Chapter 17 - Chemistry
advertisement

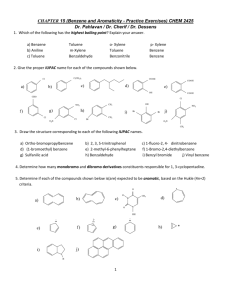
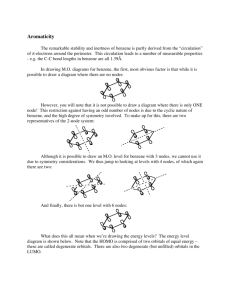
Chapter 17 - Benzene and Aromatic compounds Benzene is the simplest aromatic hydrocarbon (arene). Nomenclature: we will not discuss nomenclature of benzene derivatives in class but you are responsible for learning how to name these types of compounds. Benzene has 4 degrees of unsaturated hence is highly unsaturated. However, remarkable changes in reaction chemistry occur when the atoms of the ring are fully conjugated. Thus, whereas unsaturated hydrocarbons, such as alkenes, alkynes, and dienes readily undergo addition reactions, benzene does not. For example, HBr H2/Pt R2C CR2 no reaction very slowly formed no reaction Diels-Alder Clearly, benzene is not a triene! In fact, a comparison of the energy of benzene with that of 1,3,5hexatriene (which, like benzene, contains 3 double bonds but is an acyclic molecule) shows that the ring structure is substantially lower in energy (by 18 kcal/mol). The remarkable stability of benzene can be explained by the strong stabilization resulting from its aromatic character, which involves delocalization of electrons around the ring. Experimentally, the magnitude of delocalization energy, otherwise referred to as resonance stabilization (~36 kcal/mol), can be quantitatively determined by measuring heats of hydrogenation – see page 615 Aromaticity Aromaticity provides the benefits of high stability, etc. But what is required for a molecule to be aromatic? There are some simple rules: 1) The molecule must be cyclic 2) This cycle must be fully conjugated 3) The cycle must be planar – maximizes orbital overlap 4) The electrons must be able to “circulate”- hence must be delocalized 1 5) The conjugated cycle must contain 4n+2 π electrons (Huckel’s rule), where n = 0,1,2,3,4,..... If the conjugated cycle has only 4n π electrons, it is anti-aromatic, and will either be highly reactive, or will distort in order to violate one of the other rules (1-4). The Structure of Benzene, C 6 H 6 - benzene has a regular hexagonal structure with uniform C-C bond lengths of 1.39 Å (does not contain alternating single and double bonds!) Let’s look at the MO diagram for benzene: we start with six 2p orbitals hence we get three bonding and three antibonding MO’s – Figure 17.9. In drawing M.O. diagrams for benzene, the first, most obvious factor is that while it is possible to draw a diagram where there are no nodes, it is not possible to draw a diagram where there is only ONE node! This restriction against having an odd number of nodes (1, 3, or 5) is due to the cyclic nature of benzene, and the high degree of symmetry involved. Note that there are two possibilities for MO’s with 2 or 4 nodes. These MO energy levels have the same energy hence are called degenerate. Finally, only one MO energy level that has 6 nodes can be drawn. Thus, there are six MO energy levels, of which two pairs are degenerate; since there are six 2p electrons, they occupy the 3 lowest energy, bonding MO’s. Note that the HOMO is comprised of two degenerate orbitals, as is the LUMO. The inscribed polygon (also called a Frost Circle) method can be used to find the MO energy level diagram for planar, cyclic, fully conjugated molecules like benzene: the appropriate polygon (same size as the ring) is inscribed, vertex down, in a circle of radius 2β with the nonbonding line dividing the circle exactly in half; intersections of the ring with the circle will mark the positions of the molecular orbitals – see Page 628 We find that: (1) For conjugated cycles with an EVEN number of atoms, there will be a single high energy level, and a single low energy level. 2 For conjugated cycles with an ODD number of atoms, there will be a single low energy level, and a degenerate (i.e. double) high energy level – see page 629 (2) There are as many MO energy levels as there are atoms, and the rest come as degenerate pairs! (3) The “nonbonding” energy level runs through the middle of the diagram. Other Examples of aromatic compounds: i) pyridine, furan, pyrrole, thiophene, etc. ii) cyclopentadienyl anion; cycloheptatrienyl cation (C7H7+, tropylium ion): cyclopentadiene has a pKa of 15 and is 20 orders of magnitude more acidic than pentadiene; cycloheptatrienyl cation is remarkably stable compared to other carbocations; its salt appears to be indefinitely stable under normal conditions Let’s consider the MO diagram for the cyclopentadienyl anion (Cp-) – page 629 (odd number of atoms): 1) a single low-energy level, a degenerate high-energy level. 2) 5 carbons, minus the two high-energy levels and the one low energy level leaves TWO, which must come as a degenerate pair. 3) The nonbonding level runs through the middle of the diagram. 4) The six electrons fully occupy the set of 3 bonding MO’s and no antibonding or nonbonding MO’s are occupied, as in benzene. iii) Polycyclic aromatic hydrocarbons – which result from “fusing” benzene rings together – are also aromatic. Examples include: naphthalene, anthracene, and pyrene It is also possible to fuse rings of different sizes together – you should think about whether these would be aromatic or not! Think of C60, is it aromatic? Examples of anti-aromatic compounds: i) Cyclobutadiene (4π π electrons), on attempted preparation, rapidly dimerizes to give a nonconjugated molecule: (what sort of reaction is this?) 3 When a cyclobutadiene derivative that was too sterically hindered to react was made, it was discovered that the cycle had distorted from the square arrangement to a rectangular one– in this instance, by strongly localizing the bonds, and violating rule 4. What does this distortion do to help out with the half-filled shell? If we look at the orbital energy levels, we see that the distortion removes some of the orbital degeneracy (by lowering the molecule’s symmetry). Both electrons populate the lower energy level: Notice that this leaves a HOMO and LUMO that are VERY close in energy – highly conjugated antiaromatic cycles which distort in this way are often highly colored, because of the closeness of these two energy levels (See discussion of UV-Vis spectroscopy, Chpt 16). ii) Cyclooctatetraene: This molecule contains 8 (4n where n=2) electrons, and is antiaromatic. Let’s consider the MO diagram for cyclooctatetraene (COT) – draw frost circe (remember, even number of carbons): 1) a single lowest energy level, a single highest energy level. 2) That leaves 6 more orbitals - arranged as degenerate pairs. 3) Filling in the 8 electrons from the bottom up leaves the nonbonding HOMO half-filled. In order to avoid this high energy situation and minimize both angle strain and torsional strain, it distorts by bending (or folding) into a tub-shape: 4 We’re now violating rule #3 (and 4), and thus avoiding the aromaticity question altogether. Is there a way to make anti-aromatic molecules aromatic? Of course simply by adding or removing electrons! For example, if cyclooctatetraene is reduced (electrochemically, or with a metal like K), it easily picks up two electrons. The resulting dianion is planar, and aromatic (10 πelectrons). Similarly, cyclopentadiene (non-aromatic) is very easily deprotonated to form cyclopentadienyl anion (6 π-electrons). Annulenes: Aromatic systems that are not comprised of benzene rings (often called non-benzenoid aromatics) are a fascinating area of chemistry. These large-ring compounds are called annulenes. One of the most significant members of this family is [18]annulene (the number in brackets denotes the number of carbons in the ring and, usually, the number of π electrons): [18]annulene is a planar, fully aromatic compound. The ring is just large enough that the hydrogens sticking into the middle of the ring do not interact to twist the molecule out of planarity. In order to avoid these types of interactions, we also study dehydro annulenes (like hexadehydro[18]annulene, above). These molecules have all internal hydrogens removed, and in this case, also form aromatic systems. Antiaromatic annulenes also exist, such as the dehydro[12]annulene shown below. These compounds are usually easily reduced by two electrons to form aromatic ions. 5