A HACCP program for raw, cultured penaeid shrimp
advertisement

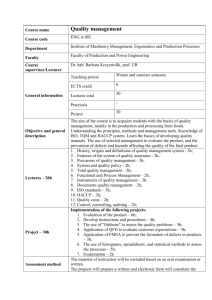
FLSGP-R-95-001 C3 FLORIDA SEA GRANT COLLEGE PROGRAM E/TP-1 A HACCP Program for Penaeid Shrimp Page218 A HACCP PROGRAM FOR RAW, CULTURED PENAEID SHRIMP LOAN COPY ONLY CIRCULATINGCOPY W. Steven Otwell ¹ and George J. Flick, Jr. 2 1Food Science and Human Nutrition Department, University of Florida, Gainesville, FL 32611 USA 2 Department of Food Science and Technology, VPI and State University, Blacksburg, VA 2406I USA ABSTRACT Shrimp continues to represent one of the safest forms of muscle protein consumed in the world, yet as for all seafoods, they will be subject to additional regulatory scrutiny through increasing use of Hazard Analysis and Critical Control Point (HACCP) programs for product safety. This example of a HACCP plan for cultured penaeid shrimp is recommended for consideration by producers, processors and importers of raw, fresh and frozen, shell-on or peeled shrimp tails. For this product form the primary critical control point is product receiving with a distinct critical limit for sulfite residuals. This plan is complimented by reference to appropriate Total Quality Assurance (TQA) programs to guide production practices and a sanitation control plan for in plant operations. Completion and implementation of these plans remains a company responsibility with additional and new record keeping requirements. The anticipated benefits are compliance for international commerce, less regulatory scrutiny per firm, consumer/buyer confidence and market access. INTRODUCTION Shrimp continues to represent one of the safest forms of muscle protein consumed in the world. Amongst seafoods, it is possibly the least problematic product in terms of reported illnesses per volume consumed. This situation was recently evidenced in the United States by the National Fisheries Institute NFI 1989). National Academy of Sciences (NAS 1991). and FDA’s Fish and Fishery Products Hazard and Control Guide (FDA 1994draft) and HACCP proposal (FR 1994). Collectively, these publications list a variety of potential health hazards that could be associated with shrimp consumption. but actual reported illnesses or outbreaks are rare and usually involve mishandling or crosscontamination in retail/food service settings or home. No doubt, shrimp are comparatively much safer than fish or chicken which have been estimated to cause 1 illness per 5,000,000 or 25,000 servings, respectively (Otwell 1989). Despite this safety record, shrimp and all other seafoods will continue to be subject to increasing scrutiny for product safety and quality. The motivating factors include more health conscious consumers and increasing competition for international commerce. This situation involves all aquatic food products, be they harvested, cultured or fabricated. The current, dominant regulatory response is hazard analysis and critical control point surveillance. HACCP regulatory programs exist in Canada, they are being formulated and demanded about the European Union, and they were recently proposed as a mandatory program in the United States (FR 1994). This brief HACCP plan for raw aquacultured shrimp illustrates some of the basic HACCP concepts and offers one initial approach for commercial and regulatory experience. Typical shrimp aquaculture and related raw product processing operations should not have difficulty with the implementation of basic HACCP programs. Initial confusion can be resolved with proper training to understand the basic principles and program expectations. Likewise, the perceived nuisance for extra monitoring and record keeping can progress to better plant management and employee commitments. HACCP CONCEPT HACCP is not a new concept. As a preventative maintenance program it has been used by various technical operations and food processing facilities for well over 30 years (NAS 1985, FR 1994). It simply involves a pre-planning procedure to fully outline an operation, noting potential hazards that could occur and identifying control points to prevent, minimize or correct hazards. Record keeping is an essential part of the program to monitor for practice. consequences and trends. In most instances shrimp processing firms have intuitively practiced HACCP activity, but they have lacked the specified monitoring and record keeping procedures. As introduced, HACCP was a management style adopted by individual company decisions. but the U.S. FDA’s recent proposal (FR 1994) would make HACCP a mandatory requirement for all seafood processors and domestic importers. FDA’s mandatory HACCP proposal is based on the basic principles best outlined by the National Advisory Committee on Microbiological Criteria for Foods ( NACMCF 1992). This selected committee recommended use of 7 steps in planning and monitoring the HACCP program (Table 1). FDA’s initial HACCP proposal deleted steps for corrective action and verification. Interpretation and use of these HACCP principles can differ by firm, regulatory agency and country. For example in the United States. FDA’s proposed program mandates the “shalls” for HACCP plans with steps for seafood safety, while recommending the “shoulds” for the various attributes of product quality and product integrity (economic fraud). The “shalls” are the enforceable part of FDA's HACCP program. In contrast, another federal agency. the Na- C.L. Browdy and J.S. Hopkins. editors. Swimming through troubled water, Proceedings of the special session on shrimp farming. Aquaculture ‘95. World Aquaculture Society. Baton Rouge. Louisiana. USA. Otwell and Flick Table 1. Seven basic principles or steps for a HACCP program (NACMCF, 1992). Steps Procedure Conduct a hazard analysis to determine potential safety problems associated with the seafood processing operations from production through final product commerce. Page 219 Table 2. A condensed version of FDA’s proposed HACCP obligations for U.S. based importers of fishery products (FR,1994) 1. Importers shall have their own HACCP plans for products while in their control. 2. Importers shall have HACCP plans on file for each of its foreign processors. 3. Importers shall take actions to ensure the products imported were produced under the HACCP plan on file and subject to FDA specified sanitation controls. Such steps may include, but would not be limited to: Identify critical control points (CCP) in processing Establish critical limits (CL) for each CCP. Establish monitoring requirements for each CCP and CL. Obtain foreign processors HACCP records Obtain ‘certification’ from foreign government authorities that the processor compiles with HACCP C. Regularly inspect the processors operations for HACCP compliance. d. Periodic end product testing. e. Other measures . . . Establish corrective actions to be taken when monitoring indicates a deviation from the CL. Establish effective recordkeeping procedures. Establish a HACCP verification procedure. tional Marine Fisheries Service in the U.S. Department of Commerce has established a voluntary, fee-for-services HACCP program that addresses both product safety and quality through all 7 basic steps (FR 1985). Both federal programs are good, but they should be distinguished. FDA is the primary food safety regulatory authority in the U.S. governing all domestic seafood production, processing and importing. They are complimented by the voluntary NMFS program which is often used by buyers and sellers to assure quality and safety in purchase. Further explanation of the respective programs is beyond the intent of this text, but additional information can be obtained from their headquarters; U.S. FDA-Office of Seafoods 200 C Street, SW Washington, DC 20204 National Marine Fisheries Service 1335 East West Highway Silver Spring Metro Center Bldg., Rm 6140 Silver Spring, MD 20910 In brief, aquacultured shrimp imported and/or processed in the U.S. will be subject to FDA’s proposed mandatory HACCP requirements. The final rule is expected in summer 1995, and compliance will follow through 1996 and 1997. Many firms are not waiting for a final rule. They reason that experience with HACCP implementation before the expected compliance dates could offer marketing advantages. Canada and some European nations are currently requesting evidence for HACCP practice. As proposed (FR 1994),U.S. importers would be required to have 4. Importers receive product from a country with an active memorandum of understanding (MCU) with FDA per HACCP. 5. Importers encourage foreign processors to obtain HACCP training per FDA specifications. on file the HACCP plans of each of its foreign processors. Proof for a valid and equivalent HACCP program could involve a variety of methods, including active participation by the respective foreign authorities (Table 2). Obviously, the time for HACCP planning has arrived. HACCP PLAN Raw penaeid shrimp, as the primary product form for most cultured shrimp, is used to illustrate a possible HACCP plan following the basic principles in Table 1. The final product form is frozen, shell-on shrimp with or without heads. This example only features product safety in compliance with FDA’s mandatory proposal (FR 1994). The plan will be an actual written document that identifies the firm, products of concern, potential hazards, critical control points, critical limits, monitoring procedures and associated records. Establishing a plan is a company responsibility. FDA does not plan any pre-approval process. Compliance will rely on company performance evidenced through routine regulatory activity. The HACCP document along with progressive records would represent a firm’s HACCP program. The document should list the firm’s address (location), telecommunications and key per- Page 220 sonnel (i.e. owners, plant managers, HACCP coordinator, etc.). HACCP plans and records will require signatures denoting maintenance by the responsible persons identified in the plan. Likewise, each firm should employ at least one individual who has successfully completed a prescribed course of HACCP instruction. The educational requirements are being addressed by a recently formed ‘Seafood HACCP Alliance for Education and Training’ initially funded by the National Sea Grant College Program (1994). This project involves a partnership of FDA, state agencies, industry and academic expertise. The specific training requirements are expected to be released with FDA’s final HACCP rule. A HACCP Program for Penaeid Shrimp RAW FRESH/FROZEN CULTURED PENAEID SHRIMP Production / Processing Flow - Diagram -RECEIVING POND HARVEST (TQA) - PROCESSING optional weigh I Transport (Chilled) I Off-Loading (*) I De-Icing / Washing 1 Step 1. Hazard Analysis The FDA does not require an illustrated flow-diagram of the production and processing operations. This approach is helpful for identifying potential hazards and critical control points. The process in Figure 1 yields fresh or frozen raw shrimp with shellon. Options include heading. Mindful of potential health hazards, the firm must list possible and reasonable problems that could occur in this process. FDA has drafted a Hazards and Controls Guide to help identify potential problems (FDA 1994draft). For raw aquacultured shrimp this guide lists three potential hazards: 1) chemical contamination; i.e. pesticides, industrial chemicals, etc., 2) food and color additives, and 3) aquaculture drugs. Many other concerns such as temperature abuse in processing, microbial contamination, decomposition and filth should also be considered as hazards. The first draft of FDA’s Hazards and Controls Guide (FDA 1994) does not list Salmonella, Vibrio or Listeria as hazards on raw cultured shrimp for critical control point monitoring in raw cultured shrimp processing. These potential bacterial pathogens would still be subject to regulatory scrutiny through import sampling. FDA reasons these pathogens are destroyed by cooking and can be prevented by proper sanitation control procedures. For this reason FDA’s initial mandatory HACCP proposal requires a written sanitary control plan with appropriate monitoring. This mandate is basically record keeping for traditional GMP’s (“good manufacturing practices”, CFR 1986). The primary safety hazards are addressed by critical control procedures. The distinction between critical control points and sanitary control plans is more obvious in daily practice and proposed record keeping procedures. Step 2. Critical Control Points Table 3 is the part of a typical HACCP plan that identifies the critical control points (step 2) and progresses through the setting of critical limits to listing of the records to be kept. By reviewing the flow-diagram it should be obvious that prevention of the three potential product safety hazards can all be controlled optional weigh I Culling | I optional heading Grading I I I optional heading I Weigh / Pack / Label - STORAGE Refrigerated Storage (*) I Frozen Storage (*) - SHIPPING Transport Transport (TQA)-subject to total quality assurance plan (*) - critical control point. Figure 1. Flow - diagram for processing raw cultured penaeid shrimp to produce fresh and frozen shell-on product. or monitored at harvest and receiving. It is best to prevent or eliminate these problems before they enter processing. If these problems are discovered during or after processing, then corrective actions and possible rejection can be more difficult, less likely and more costly. Critical control points are located at any point where hazards need to be either prevented, eliminated or reduced to acceptable levels. All significant hazards identified in step 1 must be addressed by an effective critical control point. Identifying and locating these points requires an understanding of basic food safety and the processing operations. For this reason, training is a mandatory part of FDA’s proposal. Step 3. Critical Limits In many instances, the critical limits or permissible levels for certain processing conditions, various chemical residues and microbial concerns have been established by previous regula- Otwell and Flick Page 22 1 Table 3. Raw cultured Penaeid Shrimp HACCP Plan: Steps 2 through 6. Safety Hazards Critical Control Points 1. Chemical Critical Limits Monitoring Corrective Action Record Product receiving from pond TQA Specific analysis as necessary if deviation from TQA Reject product with contamination or unapproved chemicals TQA & any analytical work, NUOCA Product receiving from pond Preservative: bisulfite residual, 100 ppm edible portion Residual screening, periodic and as needed, 3 steps: a. Sulfite test strips b. Malachite green test c. M-W verification (AOAC process) outside lab Reject product over 100 ppm sulfite residual on edible portion Progressive test results per pond harvest 3. Aquaculture Drugs Product receiving from ponds TQA Specific analysis as necessary if deviation from TQA Reject product improperly treated or containing illegal drug residuals TQA and analytical results, NUOCA 4. Temperature Abuse Storage - Refrigerator - Freezer below 40°F (4.4°C) below 0°F (-18°C) Continuous Time Temperature Charts (optional periodic monitoring) Examine for acceptable product and reduce product temperature Time Temperature chart or Periodic Records. Periodic Calibrations Contamination 2. Food Additives TQA NUOCA - Total Quality Assurance program for culture production - Notice of Unusual Occurrence and Corrective Actions tions (FDA 1994-draft), but other hazards may require special consideration. Without previous approvals, established use or designated action levels. processors should consider zero critical limits for certain pesticides and drugs. For example there is a zero tolerance for chloramphenicol in aquacultured shrimp. With the tendency for introduction of new pesticides and antibiotics through water and feed, shrimp processors should monitor on the side of caution. Additional information on legal drugs and pesticides for aquaculture use can be obtained through the FDA Center for Veterinary Medicine, Office of New Drug Evaluations in Rockville, Maryland and the FDA Office of Seafoods in Washington. DC. Their roles and activities are explained in a useful publication produced by the USDA (1992). Critical limits must be set as the guidelines for critical control point compliance. Effectiveness and acceptance of a HACCP plan rest with current and appropriate critical limits. a total quality assurance (TQA) plan for the entire production procedure (Table 4a). The TQA specifies how and when all approved chemicals are used, throughout all stages of pond production. Any deviations should be justified and recorded. Unintentional deviations may require water and product analysis by an appropriate lab to assure compliance with the critical limits as a declaration of the companies commitment to product safety before processing. Good TQA examples can be obtained from the Catfish Farmers of America (1993) and the US Trout Farmers Association (1994). Their programs feature standards for farm management practices, water quality. broodstocks, feeds and feeding, and animal health, including use of medication. All TQA participants agree to maintain records for medication and chemical applications. Their agreements are a voluntary pledge that can be substantiated with a ‘verifier’ with appropriate professional experience. These records can supplement a processors’ HACCP records. Step 4. Monitoring The HACCP plan should list the actual procedures to be used in monitoring the critical control points to assure the shrimp stay within the stated critical limits. Routine pesticide and drug analysis is not practical for most shrimp processing firms. As a preventative measure some aquaculture operations have drafted Routine procedures for field and process plant application do exist for monitoring of sulfite residuals on the edible portion of shrimp. Sodium bisulfite and metabisulfite have been used to control ‘blackspot’ or melanosis on penaeid shrimp since the early 1950’s. These reducing agents retard the natural polyphenoloxidase enzymes that create the black surface pig- A HACCP Program for Penaeid Shrimp Page 222 Table 4a. Total Quality Assurance (TQA) record for cultured shrimp production. This example is based on the recommended Total Quality Assurance programs by the Catfish Farmers of America (1992) and US Trout Farmers Association (1994). Water Quality Date and Time for Test Pond Water Analysis and Product Analysis Reason for Analysis and Reason Sampling Plan Results Medication and Chemical Application Record Date and Time for chemical use Pond Diagnosis (reason for treatment) Treatment (drug or chemical used) Rate / dose ments. Likewise, bisulfites help bleach the pigments thereby imparting a brighter, clean appearance. These actions result in sulfite residuals, both free and bound, on the shell and muscle tissue. Previous studies have concluded the original sanctioned sulfite procedure for shrimp (1.25% bisulfite dip for 1 minute; Camber et al. 1957) would leave less than 100 ppm (parts per million) on the edible portion of penaeid shrimp (Finne and Miget 1985). This work substantiated FDA’s regulatory action level of 100 ppm (FR 1985 and 1988). Other countries have established sulfite residual action levels ranging from 0 to 60 ppm. Since the original sanctioned procedures were for on-vessel use, commercial practice with sulfiting agents has changed and weaker treatments can offer effective controls for cultured shrimp. The need for action levels still remains due to adverse sulfite-induced allergic-type reactions that can occur most commonly with certain asthmatic consumers. Although there are limited investigations or reported illnesses implicating sulfites as the causative agent on shrimp, the action levels are easily and often enforced. A three phase test procedure can be used for monitoring sulfite residuals on shrimp (Table -4b). Phase one is a sulfite test paper check that only indicates distinct problems or suspect product. Commercially available sulfite test strips cannot accurately measure parts per million (ppm) on edible shrimp meat, but they can reflect a general level of concern based on the intensity of color changes when touched to the meat surface. The second phase is a malachite-green procedure (DeVries 1985) that can be adjusted to measure acceptance or rejection about a set sulfite residual level. This procedure only requires a blender, balance and pre-mixed dye. Preliminary trials are required to determine the ratio of blended meat to dye that responds to a set residual limit. For an official or FDA recognized analysis, processors must finally rely on the recognized Monier-Williams procedure Applicator Date of last dose Time of last dose Withdrawal period expiration (date and time) (AOAC 1990). This procedure requires equipment and expertise beyond the practical capability of most operations. Step 5. Corrective Action FDA’s proposed HACCP program only recommends firms to pre-consider possible corrective actions in the event that a critical limit is exceeded. Pre-documented corrective action procedures are not required, but recommended. Actions can range from product rejection to various methods of adjustment or reconditioning to assure the product complies with the critical limits. All corrective actions should be recorded with subsequent monitoring to assure the product is in compliance. Specified corrective actions are a probable addition for FDA’s final rule. Step 6. Records Each critical control point and related monitoring procedure will be accompanied by an actual record of activity (Tables 4ad). The records may be continuous, i.e., temperature charts for refrigeration, or periodic, i.e., daily lot sampling. The records are the most essential part of the HACCP program. They represent the firm’s commitment to product safety, and they can be used to judge company performance. If the HACCP document identifies a certain record, then this form should be available for regulatory review. As proposed by FDA, a company’s HACCP performance will be linked with a regulatory ranking for further surveillance. An incentive for accurate and thorough records that reflect on the actual operations and product will be less regulatory scrutiny for the processing firm and/or importer. If possible all existing records should be considered for direct or modified use as HACCP records so as to minimize the Otwell and Flick Page 223 Table 4b. Typical recording form for critical control point monitoring for sulfite residuals on raw cultured penaeid shrimp. Raw Cultured Penaeid Shrimp HACCP plan Records 1. Receiving Date Pond* Sample Sulfite Residuals Test Strip Malachite Green Action M-W Key to Positive Results: Test Strip Ratings: 1. <lOO ppm (pink), 2. Suspect, 3. >I00 ppm (brick red) Malachite Green Score: 1. <l00 ppm, 2. Suspect, 3. >I00 ppm M - W (official procedure, Monier-Williams, AOAC, 1990): Average ppm for three tests /outside lab. (list name) *Sampling.- Random accumulative sample, as product is received at the processing plant. Sample initial, med and latter portion of off-loading. Total sample size 20 - 30 lbs. Subsamples for each test procedure: test strip - 6 samples; MG - 6 samples; M - W.3 samples. Samples taken per pond harvest with or without sulfite treatments. amount of record keeping. For example, an invoice or record of incoming product could also identify checks for sulfite residuals. Combining records can be useful, but it should not compromise confidential production information. FDA’s proposed regulatory concerns rest primarily with product safety. All HACCP records must be maintained or filed for one to two years if the products are fresh or frozen, respectively. Step 7. Verification FDA's proposed mandatory HACCP proposal does not require a verification plan. Verification is considered a company responsibility to assure their HACCP plan is effective. Verification can involve periodic end-product testing, in-plant and outside audits, and annual plant reviews. Verifications can also include calibrations or checks on monitoring methods or devices. Any change or addition to the processing operations would require modification of the HACCP plan and verification. FDA’s final HACCP rule will most likely include some verification requirement. The ultimate verification is an FDA HACCP inspection. SANITATION CONTROL PLAN In addition to the basic HACCP plan with critical control points for product safety, FDA has proposed that every processing firm should have a written sanitation control plan that includes adequate record keeping for performance. This requirement is one of the more controversial and debated parts of FDA’s HACCP proposal. The requirement basically addresses all “good manufacturing practices” and emphasizes certain points for particular attention. Concerns range from cleanliness and sanitation of operations, equipment, surfaces, and clothing, etc. to personnel hygiene, pest control, time-temperature controls, etc. The expectations are reasonable, but tedious record keeping requirements have been questioned. Assuming record keeping is required or generally accepted by a firm, these sanitation record keeping procedures could be segregated by plant operations managers and by time (Table 4cd). Most sanitation procedures require ‘continuous monitoring’ which could be recorded by the actual ‘begin and end’ working time of an assigned employee (Table 4c). For the more routine periodic procedures, i.e. daily, weekly or monthly activities, may require actual logs to designate completion of procedures (Table 4d). SUMMARY Despite the safety record for consumption of cultured penaeid shrimp, as for all seafoods, further regulatory scrutiny is expected in the form of HACCP programs. The safety associated with raw cultured shrimp is evidenced by the simplicity of the HACCP plan for raw cultured shrimp. Following FDA’s proposed HACCP mandate, firms can anticipate needs for TQA plans and monitoring for sulfite residuals and storage temperatures. In the United States the burden of proof for HACCP compliance is proposed to rest with the domestic importer. Processors should initiate HACCP planning to gain experience in advance of the final FDA rule expected in mid- 1995. A HACCP Program for Penaeid Shrimp Page 224 Table 4c. Partial example for a daily sanitation record for ‘ continuous items’ to be controlled in a shrimp processing plant sanitary control plan. The record is structured by plant personnel responsible for items that require continuous attention. Deviations and corrections in procedures are recorded asNUOCA’s (notice of unusual occurrences and corrective actions) and compliance is denoted by the appropriate daily signature. Any Shrimp Co., Inc. Daily Sanitation Record Continuous Items (From pre-operation review thru end of work day) NUOCA - General housekeeping to avoid clutter that hampers plant operations and sanitation. - Plant layout and general condition helps prevent product contamination and assures sanitation. - No condensation on pipes, ceilings or other surfaces that could result in product contamination. - Equipment, facilities and processing utensils in good operating condition and able to be sanitized. - All wet and dry waste materials segregated and removed from the plant into proper disposal. - Brushes, trash cans and clothes used to clean and sanitize are color coded to distinguish. - All product containers stored in clean, dry area free of personnel and product traffic, and protected from pests. - All chemicals for equipment use, pest management, cleaning and sanitizing must be stored segregated and separate from the processing area. - Convenient hand wash facilities. clean and properly equipped. - Water supply approved. - Ice supply clean and protected from contaminants due to floor traffic or equipment contact. - No worker with illness, open or infected wound will be allowed to come in contact with product or plant operations. - No worker will be allowed into the processing area without previous training for food handling and sanitation. - No personnel will be allowed in the processing area without clean garments and hair covering. - Control all personnel traffic. Only authorized personnel in processing plant operation area. Date HACCP Record Manager Date Plant Manager Otwell and Flick Page 225 Table 4d. Partial example for a daily sanitation record for ‘periodic’ items to be controlled in a shrimp processing plant’s sanitary control plan. The recorded is structured for the personnel responsible for certain items that require periodic attention at specified time. Deviations and corrections in procedures are recorded as NUOCA’s (notice of unusual occurrence and corrective action) and daily compliance is denoted by the appropriate signature Any Shrimp Co., Inc. Daily Sanitation Record “Periodic Items”(D=Daily Time; W=Weekly Time & Day) Time & NUOCA - Conduct Pre-operation review for general plant condition and sanitation. including workers. Listed time marks beginning for “continuous” records. D*= - Clean and sanitize shucking area and surfaces that contact product; tables, drains, floor, walls, utensils and equipment; At least once daily and/or before each break and at the end of operations. D= D= - Clean and sanitize the cooler / refrigeration storage. Daily for dry clean and weekly for full cleaning and sanitization. D= w= - Clean and sanitize washroom areas and facilities. D= - Inspect and clean as necessary all waste disposal areas and completely clean and wash weekly. All waste containers should be clean and sanitize daily. D= w= - Inspect plant premises for clutter and general filth that may attract pests; clean and remove excess weeds, vegetation, waste, etc. w= - Inspect all areas for presence of insects and/ or rodent and implement rodent control program. w= - Inspect lighting and ventilation for proper operation or possible product contamination. w= - Inspect all chemical storage for segregation from processing area and possible leakage or spills. D= - OTHER: * D = time of day W = time of week Date HACCP Record Manager Processors should note that HACCP regulations are not intended to replace existing regulations. In reality. the proposed program is in addition to current regulations. Imported shrimp processed under a HACCP plan are still subject to product sampling and possible rejection. particularity for decomposition, filth, chemical contaminants, microbial content, improper labeling and various economic frauds. The advantage of an effective, recognized HACCP plan is proposed to be less regulatory scrutiny. HACCP is an honor system with the reward of product/firm confidence. This concept is new, evolving and a significant challenge for international seafood commerce. Date Plant Manager LITERATURE CITED A.O.A.C. 1990. Official methods of analysis, Association of Official Analytical Chemists, 15th Edition, AOAC, Inc., Arlington, Viginia, USA. Camber, C.I., M.H. Vance, and J.E. Alexander. 1957. The use of sodium bisulfite for the control of blackspot in shrimp. State of Florida Board of Conservation Technical Series No. 20. Page 226 A HACCP Program for Penaeid Shrimp Catfish Farmers of America. 1993. Catfish Quality Assurance. Indianola, Mississippi, USA. Otwell, W.S. 1989. Regulatory status of aquacultured products p Food Technology 43(11)103-105. Code of Federal Regulations (CFR) 1986. Current good manufacturing practice in manufacturing, packing or holding human food. 21 CFR Part110, Federal Register 51(118)22475. US Department of Agriculture (USDA). 1992. Federal regulation of drugs, biologicals and chemicals used in aquaculture production. National Agriculture Library, US Department of Agriculture, Beltsville, Maryland, USA. DeVries, J. 1985. Determination of the presence of sulfite (>I00 ppm) [Malachite-Green procedure]. General Mills, Inc., Technology and Quality Control Services, Minneapolis, Mimmesota, USA Finne, G. and R. Miget. 1985. Use of sulfiting agents in shrimp - US Regulations for domestically produced and imported products. INFOFISH Marketing Digest, No. 4/85:39-40. Federal Register (FR). 1985. Notice to shippers, distributors, packers and importers of shrimp containing sulfites. US Government Printing Office, Washington, DC 50( 15)2957. Federal Register (FR). 1988. Sulfiting agents in standardized foods; labeling requirements; proposed rule. US Government Printing Office, Washington, DC 53:51062- 51065 Federal Register (FR). 1994. Proposal to establish procedures for the safe processing and importing of fish and fishery products; proposed rule. US Government Printing Office, Washington, DC 59( 19) 4142-4214. Food and Drug Administration (FDA). 1994. Fish and fishery products hazards and controls guide [draft]. FDA Office of Seafoods, Washington, DC. USA. National Academy of Sciences (NAS). 1985. An evaluation of the role of microbiological criteria for foods and food ingredients. National Academy Press, Washington, DC, USA. National Academy of Sciences (NAS). 1991. Seafood safety. National Academy Press, Washington, DC, USA. National Advisory Committee on Microbiological Criteria for Foods (NACMCF). 1992. Hazard analysis and critical control point system. International Journal of Food Microbiology 16: l-23. National Fisheries Institute (NFI). 1989. Seafood industry HACCP workshop and model tests: raw shrimp - Final report, National Fisheries Institute, Arlington, Virginia, USA. National Sea Grant College Program. 1994. Seafood HACCP alliance for education and training. National Project No. NA36RGOO70. National Sea Grant Office. Silver Spring, Maryland, USA. US Trout Farmers Association. 1994. Trout producers quality assurance program. US Trout Farmers Association, Harper’s Ferry, West Virginia, USA.