Lab Thickness of a Molecule
advertisement
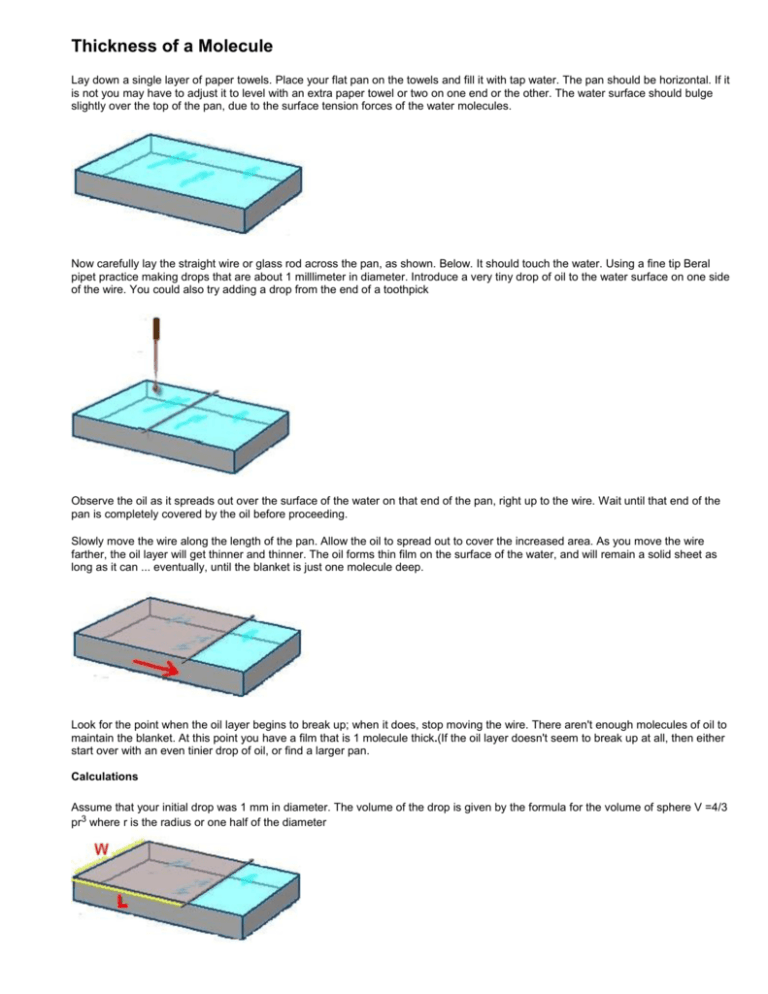
Thickness of a Molecule Lay down a single layer of paper towels. Place your flat pan on the towels and fill it with tap water. The pan should be horizontal. If it is not you may have to adjust it to level with an extra paper towel or two on one end or the other. The water surface should bulge slightly over the top of the pan, due to the surface tension forces of the water molecules. Now carefully lay the straight wire or glass rod across the pan, as shown. Below. It should touch the water. Using a fine tip Beral pipet practice making drops that are about 1 milllimeter in diameter. Introduce a very tiny drop of oil to the water surface on one side of the wire. You could also try adding a drop from the end of a toothpick Observe the oil as it spreads out over the surface of the water on that end of the pan, right up to the wire. Wait until that end of the pan is completely covered by the oil before proceeding. Slowly move the wire along the length of the pan. Allow the oil to spread out to cover the increased area. As you move the wire farther, the oil layer will get thinner and thinner. The oil forms thin film on the surface of the water, and will remain a solid sheet as long as it can ... eventually, until the blanket is just one molecule deep. Look for the point when the oil layer begins to break up; when it does, stop moving the wire. There aren't enough molecules of oil to maintain the blanket. At this point you have a film that is 1 molecule thick.(If the oil layer doesn't seem to break up at all, then either start over with an even tinier drop of oil, or find a larger pan. Calculations Assume that your initial drop was 1 mm in diameter. The volume of the drop is given by the formula for the volume of sphere V =4/3 pr3 where r is the radius or one half of the diameter Assume that the calculated volume is now filling a box whose dimensions are L x W x H, where H is the thickness of the oil blanket, or the diameter of one molecule. For example, if the volume of the drop was 1 cubic millimeter and the dimensions are 400mm and 300mm, then the following calculation shows how to solve for H: V=LxWxH 1 = 400 x 300 x H 0.00000833 = H This makes the height of the oil blanket, or the diameter of one molecule, equal to 0.00000833 mm, or 0.00000000833 meters. Given the inaccuracy of our measurement, it's probably safe to round this number to 0.00000001 meter, which is one ten-millionth , or 10-7 meter. Repeat the procedure different organic liquids available in the lab. Are there differences in the thickness of the layers that are produced





