from simple to complex: protein expression using animal
advertisement
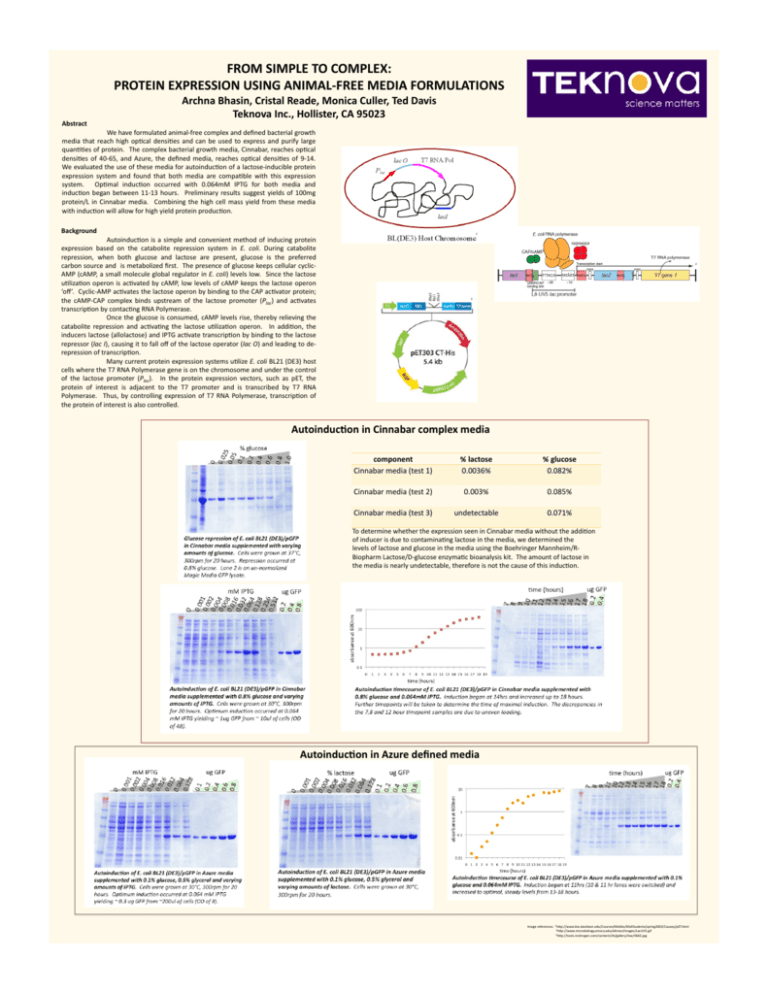
FROM SIMPLE TO COMPLEX: PROTEIN EXPRESSION USING ANIMAL-­‐FREE MEDIA FORMULATIONS Abstract Archna Bhasin, Cristal Reade, Monica Culler, Ted Davis Teknova Inc., Hollister, CA 95023 We have formulated animal-­‐free complex and defined bacterial growth media that reach high op9cal densi9es and can be used to express and purify large quan99es of protein. The complex bacterial growth media, Cinnabar, reaches op9cal densi9es of 40-­‐65, and Azure, the defined media, reaches op9cal densi9es of 9-­‐14. We evaluated the use of these media for autoinduc9on of a lactose-­‐inducible protein expression system and found that both media are compa9ble with this expression system. Op9mal induc9on occurred with 0.064mM IPTG for both media and induc9on began between 11-­‐13 hours. Preliminary results suggest yields of 100mg protein/L in Cinnabar media. Combining the high cell mass yield from these media with induc9on will allow for high yield protein produc9on. Background 1 Autoinduc9on is a simple and convenient method of inducing protein expression based on the catabolite repression system in E. coli. During catabolite repression, when both glucose and lactose are present, glucose is the preferred carbon source and is metabolized first. The presence of glucose keeps cellular cyclic-­‐ AMP (cAMP, a small molecule global regulator in E. coli) levels low. Since the lactose u9liza9on operon is ac9vated by cAMP, low levels of cAMP keeps the lactose operon ‘off’. Cyclic-­‐AMP ac9vates the lactose operon by binding to the CAP ac9vator protein; the cAMP-­‐CAP complex binds upstream of the lactose promoter (Plac) and ac9vates transcrip9on by contac9ng RNA Polymerase. Once the glucose is consumed, cAMP levels rise, thereby relieving the catabolite repression and ac9va9ng the lactose u9liza9on operon. In addi9on, the inducers lactose (allolactose) and IPTG ac9vate transcrip9on by binding to the lactose repressor (lac I), causing it to fall off of the lactose operator (lac O) and leading to de-­‐ repression of transcrip9on. Many current protein expression systems u9lize E. coli BL21 (DE3) host cells where the T7 RNA Polymerase gene is on the chromosome and under the control of the lactose promoter (Plac). In the protein expression vectors, such as pET, the protein of interest is adjacent to the T7 promoter and is transcribed by T7 RNA Polymerase. Thus, by controlling expression of T7 RNA Polymerase, transcrip9on of the protein of interest is also controlled. 2 3 AutoinducRon in Cinnabar complex media component Cinnabar media (test 1) % lactose 0.0036% % glucose 0.082% Cinnabar media (test 2) 0.003% 0.085% Cinnabar media (test 3) undetectable 0.071% To determine whether the expression seen in Cinnabar media without the addi9on of inducer is due to contamina9ng lactose in the media, we determined the levels of lactose and glucose in the media using the Boehringer Mannheim/R-­‐ Biopharm Lactose/D-­‐glucose enzyma9c bioanalysis kit. The amount of lactose in the media is nearly undetectable, therefore is not the cause of this induc9on. AutoinducRon in Azure defined media Image references: 1hdp://www.bio.davidson.edu/Courses/Molbio/MolStudents/spring2003/Causey/pET.html 2hdp://www.microbiology.emory.edu/altman/images/LacUV5.gif 3hdp://tools.invitrogen.com/content/sfs/gallery/low/4665.jpg





![Lac Operon AP Biology PhET Simulation[1]](http://s3.studylib.net/store/data/006805976_1-a15f6d5ce2299a278136113aece5b534-300x300.png)

