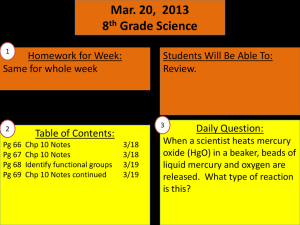
TOPIC LIST FOR SEMESTER 1 CHEMISTRY FINAL EXAM YOU
advertisement

TOPIC LIST FOR SEMESTER 1 CHEMISTRY FINAL EXAM YOU MAY DESIGN AN INDEX CARD THESE ARE THE CONCEPTS COVERED ON YOUR SEMESTER EXAM. YOU ARE TO RESEARCH/REVIEW EACH CONCEPT AND WRITE ABOUT EACH ONE. SIMPLY DEFINING IS NOT ENOUGH. THAT WILL ONLY EARN YOU HALF CREDIT ON EACH. YOU SHOULD EXPLAIN EACH CONCEPT THOROUGHLY FOR FULL CREDIT. SIMPLY OPEN UP YOUR NICELY ORGANIZED BINDER TO EACH OF THE FOUR UNITS BELOW AND THE MATERIALS SHOULD BE AT YOUR FINGERTIPS! AS YOU STUDY THEM AND LEARN THEM PROFICIENTLY, CHECK THEM OFF AND HAVE YOUR PARENTS QUIZ YOU IN THE DAYS LEADING UP TO THE EXAM. DOWN BELOW IS A SET OF REVIEW PROBLEMS. DO THEM AND YOUR GRADE WILL DEFINITELY IMPROVE. AVOID THEM AND YOUR GRADE WILL LIKELY BE LOW. UNIT ZERO What is Chemistry? Matter Mass Physical vs. Chemical Properties Mixtures Phyiscal vs. Chemical Changes Law of Conservation of Mass Reactants vs. Products Scientific Notation Precision vs. Accuracy Metric System Prefixes Density Problems Temperature Conversions PROBLEMS: CHP 1: 27, 29, 31, 32 CHP 2: 74, 75CD, 76CD, 77HI, 78CF, 79DF, 80DE, 83F CHP 3: 37CDE, 39CDE, 42,45, 49, 52, 61, 66 UNIT ONE What is an atom? What are atomic mass units? What are Subatomic Particles Experiments that led to subatomic particle discovery (both protons and electrons) Atomic Number Atomic Mass Isotopes Mass Number Calculation of neutrons using mass numbers UNIT TWO The electromagnetic spectrum Waves, Frequency and Amplitude Periodic Table basics Aufbau Sequence Hund’s rule Pauli Exclusion Principle Electron Configurations of atoms and ions Regions 4 Trends – (How are they defined and how do the trends vary around the periodic table) Group Names Reactivity PROBLEMS: CHP 5: 30ab, 33, 38, 44, 70, 78C, 80DF, 81BE, 93, 94 CHP 6: 30, 48, 52, 57, 60, 64, 68 UNIT THREE Ionic Compounds How are names written (full procedure) How are formulas written (full procedure) Octet Rule Metals PROBLEMS: CHP 8: 51, 52, 56, 61DE, 62, 63, 67CD, 69, 74CE, 75CE Radioactivity Nuclear Equations PROBLEMS: CHP 4: 43, 46, 57, 60C, 63, 64C, 65C