Lecture Notes - Department of Physics, HKU
advertisement

Æ ! "#$ "%&&
'(! )*+, ))
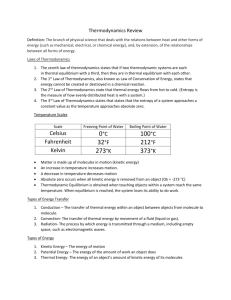
** ! - "!.. ( !.. /
* 0
* 1 *2 ( * 3 (
4 * 1 4 * 5 , , )
1 * 2
4 * * 24
*
** *
*
* 62* 7
2
1 * 4* 1 (
* * *
4* * 1 *
* 1 , 4** 0 * * *(
* ,
*
1 * 2
1 * * )* * 1 * 8/ 3 * 0
* * * *
* *9
.: * * 8 , * 2 )
8:9 *
$.(* ;< 8%:99
.: 1 *2 ( 2** =* A /
;2
$ (
<
( ; !,,"<
' B
;C +
D !,,!<
*( C '. ;&
. B
1" ((
%
(
<
D. C + C.(( ;/&
!,,- ( (
<
A( A ;(
B<
E D ;A
+
0 !,,,<
! F( !! (
8
( (
8( !# (
!- 1( A :&(( (
#
0
"
,
,
!
#
$
!,
!!
#
#!
##
##
! :.(; B
( 3. 6 6 (
8 (
:.(; E 3. :.(; /
3. 1
(
6 :.(; 3. # '
( #! C
3
6( # $
- 9(
(
-! E(
6
*9( -# G
6 (
-- ( E( % &&
! %
%A .
( (
6 !
! B
(
6 !0
! E((
6
(
!0
!! (
6
(
!"
# E A 6 (
!
' & ( $ 5
(
6 ( $! *(. *
$# ( $- 8(
6 ( $ /. (
(. ) * $
0 ( 6 0! ( 6 6 *
0# ( 6 ( 6 (
0- E( 6 8
* #
###
#
#0
"
#
-!
--$
+ ,
#"
" ((
.
( (( (
,
"! (
*(. 8
* " /? ! ((
(
:.(; ! 3. ((
# A
( - ((
(
6 *
%?
*
6 * $ :&?
*
((
((
0 :&?
*
((
((
((
(
- , 5
(
,! 1 ( 8
(
,# 8(
6 1 ( ,- E(*
( 6 *9( %
#
$,
$#
$$
$"
)
0!
00$
00
./ ,
0
( $
0
+' * (( 6 ! +' * 8
* 6 ! !! /. # +'& ( - +' '
(
((
(
(
$ 8(
8 6 0 ((
% " 8(
6 % 8(
8 ( +",
"
"
"#
""
"$
"0
""
"
1
0
"#
! ( ( 6 (
!! E A 6 8(
6 $
21
# :.(; 3. 6 G
8 8
((
#! 8
((
( %(; 6 ## %7( 6 %(; ((
#- 8
((
6 ( 6 # 8
((
((
#$ ((
6 (
( #0 % (
6 (
( (
"+
"
"
,,
,,
,
,!
14 THERMODYNAMICS
103
14.1 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 103
14.2 Macroscopic Description of Matter . . . . . . . . . . . . . . . . . . . . . . . . . 104
14.2.1. State variables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 104
14.2.2. Temperature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 105
14.2.3. Phase changes, phase diagrams . . . . . . . . . . . . . . . . . . . . . . . 106
14.2.4. Ideal Gas Processes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 108
15 Heat, the First Law of Thermodynamics
113
15.1 Work and Heat . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 113
15.1.1Work done on/by ideal-gas processes . . . . . . . . . . . . . . . . . . . . . 114
15.1.2 Heat . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . 115
15.2 The First-Law of Thermodynamics . . . . . . . . . . . . . . . . . . . . . . . . . 115
15.3 Thermal Properties of Matter . . . . . . . . . . . . . . . . . . . . . . . . . . . . 116
15.3.1 Heat of transformation . . . . . . . . . . . . . . . . . . . . . . . . . . . . 116
15.3.2 The specific heat of gases . . . . . . . . . . . . . . . . . . . . . . . . . . 117
15.3.3 More on adiabatic process . . . . . . . . . . . . . . . . . . . . . . . . . . 118
16 From Micro to Macro, Entropy of the 2nd Law of Thermodynamics
119
16.1 The Kinetic Theory of Gases . . . . . . . . . . . . . . . . . . . . . . . . . . . . 119
16.1.1 Maxwell speed Distribution . . . . . . . . . . . . . . . . . . . . . . . . . 119
16.1.2 Mean Free Path (MFP): the average distance between collision . . . . . . 121
16.1.3 Microscopic origin of PRESSURE . . . . . . . . . . . . . . . . . . . . . . 121
16.1.4 Microscopic View of TEMPERATURE . . . . . . . . . . . . . . . . . . . 121
16.2 Thermal energy and specific heat . . . . . . . . . . . . . . . . . . . . . . . . . . 121
16.3 Thermal interaction & Heat . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 123
16.4 Irreversible Processes, Entropy and the 2nd Law of Thermodynamics . . . . . . . 124
17 Heat Engines & Refrigerators
125
17.1 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 125
17.2 Heat to work and work to heat . . . . . . . . . . . . . . . . . . . . . . . . . . . 125
iv
17.3 Heat engines and refrigerators . . . . . . . . . . . . . . . . . . . . . . . . . . . . 126
17.4 Ideal-gas engines and refrigerator . . . . . . . . . . . . . . . . . . . . . . . . . . 127
17.5 The Carnot Cycle and the limit of efficiency . . . . . . . . . . . . . . . . . . . . 129
iv
1 ( ?(
(
( 6 ( ( *
&
B A (
H3/ I
6
H3/ I
%
H3 / I
( H3/ I
B ?(
( * 6 ( ( * ? ( (( 6 6(
0&
+ '. 6 ?
( ' *9( (
/ 6 (
( *9( 6 ( *9( ( 6 ( (
*
!
H I J H IH IH I
H3/ I J HI H3/ I H3I
J HI H3I H/I
J J ! J 0&
/ 6? 6 8
*(
6 ( ( 6 (( (
7 ((
( ( * (
6 ( 6 J <((= G ( 5 ( '. (( H I J H/ I H
I J H/ I
*
G
H/ I J HI H/ I J H / I
K J , ! J J J !
= 1 8( *( ( (
( ( * .
y
vy
v
θ
vx
x
−v
( A&( 6 8( ( &( 6 8( / ( 6 ( 8( ( * .
J J K *= #
-
y
a+b
a
b
x
= y
( (
*( (
? ( (
( ( 6 J αa
a
x
= L
L9 ( ( 8( 6 (
.
8 ( 6 ( L
J L9 J / J L
K L9
+
( ( 6
J L
K L9 J L
K L9
K J < K =L
K < K =L9
J L
K L9
y
vxi
vx
v
vy
j
vy j
i
(
8(
& 8( ( * ( (
6 (
( (
x
y
<=
M
∆r
r(t1)
r(t2 )
x
(
8(
J <=L
K <=L9
J < = < =
18 8
( (
M J < = < =
J
M
6 ( (
(8 M 5
(
M , ( (( 8
( (
(
M J <= <((
( 8 <==
J M
3
'.
( 8 (
(
M J < = < =
J
M
M J <= J <=
J M
6 . (' *( 8
( (
. ('
*( ((
8
( (
% 9(
(
J L
K L9
<= J < =L
K H< = y
v(t1)
v0
θ
r(t1) a(t1)
x
L
! I9
<= J < =L
K H< = IL9
<= J L9
1 A /.
( (. ( ' 8(
.
( 6 $
y
way
up
t=0
just before
touching
ground
way
down
max.
height
v=0
a=g
a=g
v<v0
v=v0
a=g
a=g
ymax
y
a=g
v<v0 y
0
v=v0
decelerate
accelerate
/' . (
8 (
J
J
J J J J K J ((
/ (
1 *(
(( ( (
(
( J ,
J < J ,= J J K
J J J < = J !
/ (
*(
(( J , <!=
K J ((
< J ,= J J ,
1( A
( !
J J , J , J J <= J
/
6 (
( J ,
J J , !
! J ! J ! <
J ! =
<!!=
0
a
t
−g
v
v0
t
v0
y
v02
2g
v0 t− 1 gt 2
2
2v0
g
v0
g
t
( ( ( 6 .
( ( (
6 ( (
8 1
(
J J (
8 ((
y
a=−g−kv
y(0)=h
0
J J K8
K8
(
"
J <=
<=
J <= <= J J ((
J K < = J K J J . J J < =
1( J , <,= J , J , J J < K = J < =
J
< =
v
g
k
t
< J J
= K K
J ((
1( J , J J K J K J KK
J K < =
!
6 ( ( 6 (
* ( ( * .
8 ( (( 6 (
6 ( * ( ( ( ( ( (N 6 ( * 8 .
( 8
( ( .
' 8
.
( (( (( 8
(
+( ;6;2
( 6 7( '
6 6
& %((
6 A
6O
& 8
((
6 & E( 6
& +' 6
"# # 6 6 + . *8 ( (
6 *9(2
%(; 6
; 6 8
.
( .
( ( ( E
rmS’
rmS
S’
rS’S
v S’S
S
¼
,
. & (
8( 6 ( *8 6 ( (
& (
8( 6 ( *9( *8 6 ( (
& (
8( 6 ( *9( *8 6 ( 8
¼
¼
:(
(( J B ( 8( ¼
¼
J K J K J K ¼
¼
¼
¼
¼
¼
. & 8
( 6 ( *8 6 ( (
& 8
( 6 ( *9( 6 ( (
& 8
( 6 ( *9( 6 ( 8
: (
( . (
. 6 .
( *8(
( (
¼
¼
$ !
6 ( ( 6 (
*9( ( ( 6 .
* ? ( ( ( 6
6 ( *9( (
((( 6 ( 6 ( *9( (( J . (
*9( (
G
( 6 6 :.( J H3/ I
:.( 6 6 ( 6 ( ' *9( (( .
( ( *9( .
(
(
6 % !
1 * A( 6 ( * ( * A( 6 ( 5(
/ (. 6 ? ( *( ( (
F −F
& # ! '
(
1 .' '
( 6 J -!' 6( 6 ( ( ( 6 J -' ( ( ( P .
(( 6
(
/
.' ( .
( 6 J #,: B
( (
6 ( 6(
( 6 A( * ( ( !
a
a
R1
m1
m2
P1w
m1
m1 g
R3
R2
R2
m2
m2 g
. & (
6 ( & (
(
*(. & (
6 ( :( (( J J 8(
(
<
J ,=
/'
( (
8 G
:.(; ! . . 5 ( ?(
6 (
J <#=
J <#!=
!
E*(
(( <#!= ( <#= . (
J J K J J
K 3 ( ((
( ( (
6 . ( (. ( (&
* (
(( ( ( 6 6( .
(
6 6( J 3
6( (
*( 8 . .
( (( < 5
*.=
T2
11
00
00
11
T2
T2
T1
T1
T1
mg
Mg
11
00
00
11
T1
mg
Mg
/'
. (
8 E
J , ( ?(
6 (
8 *
J , J J , J < K =
: ( ( 6 = J , ,
!= J , J ,
#
! 3
6( ( . .
( (
:( (( ( * (
8 6 (
/6 ( ?(
6 (
.
8 *
J J < K =
J J < K =< K =
:( (( (
( (( ( 8
< J ,=
1 (
6( (
. . 6 6 ( ;6; . (.
( 6( P . . ( 6( /
:/ 6 *( 6
0&
( ( ( (
.
( .
-&' . . 6 (
.
(( (
6 #,, :
*
1
( :.(; .
J /6 ('
(
8 (
( (
6 ( ( (
6 ( . ( ( *
J . ( 6 ( .
:. ( 8 (( ( A
(
6 ( J #,, : C (
(
6 ( . 8 *
H<- '=<" = #,, :I
J
J
J ## - '
) *
y
v0
θ
1( J ,
x
J J,
J J 3( ( ( @( 8(
(
6 ( *9( (
C
@( (
J,
J J J ((
J J <= J 1( J ,
<= J
K J < = K -
J ((
1( J , J , J ,
<= J < =
F(
(
J J J K J ((
1( J ,
J J J <= J K <= J K K J K < = K !
!
1( J , J , J ,
<= J < = !
<= J ,
<= J <= J <= J <= J < =
<= J < = <= J L9
<= J L
K < =L9
<= J < =L
K H< = IL9
!
3( * ( (
6 P
( (
, J < = ! J , <( ( ( (
((=
J ! 3( * ( @( (
J < = J < = J
A
( J -Æ ! J !
J ((
$
$+ #+ ,)
y
R
1
0
0m
1
0
1
1
0
0
1
0
1
v0
φ
x
1( (
J , ( *( 5 .
( 8
( ((
( (
(( (( .( ( 8 6 ( *( ( ( 3( * ( (
8( 6 ( *( * ( (
8( 6 ( 1( J ,
J L9
J L9 K J (( 8(
J , J ,
J L9
<= J L9 K 1( J , J J !
<= J L9
!
1( J ,
J L9
J L9 K J J J (( 8(
J L9 K <= J L9 K !
0
1( J , J , J ,
!
<= J L9
.
(
( L
L9 (
J !L
K !L9
J !L
K !L9
. J <= J < !=L
K H< != IL9
!
<= J !L
K H ! ! IL9
(
/ ' 6 ( .
. '
1 ( *( ( J ! ( (
( 6 ( *( ( ( 2
3( * ( (
(( ( *( J !
< != J ! J &( 6 ( *( < = J !
! &( 6 ( < = J ! ! J <=
/ ( *( .
( ( 0&
/ 6.
5 . * *
(. . 6 ( ( 6 .
. 6 ( 6 !"Æ (
8
( 6 ( * J
## ( 6 J $Æ <.
( ( ( ( @(= 1( .( ( ( ( * (
' .( (
2
"
*
+ ( 9(
8 ( @( ( 6 ( ( 8
( ((
( ( (
8 / ( (
6 P
( ( ( 6.
J
J
. ( (
8
( ( 6 9(
G
(
(
.
*(
( (9( ?(
6 ( 9(
! J J < =
!
J ( ! :( (( J ( 8(
( 6 ( (
8
(
((
( ( 6 ( * ( ( *8 ?(
. 5 ( (9( ?(
<"= J !- ,,!!
J ( $Æ !<##= $Æ
/ ?(
6 ( J <( !"Æ = J ,#!
1( ( 8 6 6 .
( * ( ( . 8
,#! J !- ,,!!
$," ,,!! J ,
J $#" </ J , (
9( ( 6 ( ( ((
(= / ( ( *
J !"Æ J " !"Æ J $#" " !"Æ J 0!# 1 ( (
( (
( $#" J
J
J -0 <## = $Æ
0&
(( & * ( 6 #, "# 6 6 ( P( * (( (
( ( !, / .
( '
( * ( (( ( ( 9( !, . 1( .( (
( ( * 2
*
/ * ( ( 9(
(
.
( 8
((
(
J !, / ( !, * ( 6 E . 8
J < =
J < = ! <-=
B ( ?(
( . 8 J ! < !, = J /( !, J ( <-!=
! ( 8 ( J ! J , J # "# ( ( ?(
.
*(
0! ( K J ,
( !,
16( 8
. 8 ( J -$ ,$"!! /( J 0 #-# !,
-# (. *
& @( 6
(
y
v1
P1
θ
vp
P
P2
θ θ
θ
v2
J L
K L9
x J L
L9
r
/
?
6 8
6 ( M J ! < =
:( (( J J &( 6 8 (
J
M
J J J ,
&( 6 8 (
J
M
J J !
J
!" J J :. . (' ( ( , ( *(
( (( (
( J J
"
6 * ** 2* *
* I .
>* * ** * (* * * 2
* *
1 * * * 1 #
? 1 * * ** * I # 2
* * 2
* * 1 * y
v
a
x
r
1'
> 2* * 1* * * *) 2 * , 2 0
/ 4 * 1 * *) 4 1 4* / ,
*
*
1 * * >* 4 1 * 2 , * ** 2* * *)
* * 2 * ** 2* * *) * *
4 1 1 2 *
""
+
1 * P* * 1 * *) * * 1 * *) * % J & .
%I
2 * 4 * 1 * / * ** ,1 >* * P* * * I %. I I A
* 1* * *
* % I 2 *) < *4 4 @ *
1 2 * 2 1 1* 2 *
2 1 *
% , / / * * ** * ** 2* * *) * 2 % . %. / 4* 1 G %. H I * ;
@ * ; ) %
** * 4* * *
8 9 * 1 *(
*
8 9 * , * * 1 * - 0 *2 * 4* *
** * * 8 / * 4* 8K' K( 9 1 *M 8K K 9 1 9
K I K' J K(
8%9
I K' J K(
K
I K' J K( I K
89
K
K
K
I ' ( I K
/ * 4* *
1 *
4 , I K I K 8986 *
**! K I K 899
K
!#
/ ( 8
( J
J <L = J L K L
L
L
Q
< J J L =
J QL K QL
J L K L
<-=
<-$=
<-0=
<-"=
. 8
( ( 6 L 8
( (
6 L / (
6 ( ( 8 *
J <QL K QL =
J
<-=
L Q
L J Q
L K Q K QL K L K Q J RL K Q <L =Q K Q QL K RL K Q<L =Q
J <R Q =L K <R K !QQ=L
<-,=
. <R Q = J (
<R K !QQ= J (
0&
1 ( ( 6( !) (( @( *( ( ( .
( &
(( $") 6 ( (
(
6 ( ( ) 6
(( !") (( ( ( ( ( ( ( 6 !-
.
( / 9( 6 ( ( 6 ( .
(
#) (
8 ( ( ( (
( E. (( .
( ((
.
( ( ( ( 6 ( (
5 ( ?(
R -
J ! B
(
)
* (
6 (
?(
*
E
( ( ( ( 6 (( ( ( ( ( (
6 ( ( (
(
/ ( 6 ( 6 8 *
! < )= / (
6 ( 8 *
)
! < )= J <R Q = <-=
)
( 6( 6 %? <-= (
8 ( ( (
(
6 ( (
6 (
6 ( ( ( ((
( ( /
( ( ( 6 ( ( 6 %? <-,= ( %? <-=
Q
( J $") . 8
$ !
< )= J R )
)
J ! / (. * (
6 (
16( 5(
. 8 R -
)
?(
<,= J ) Q <,= J # )
& /
#
%A ?
*
( (* * J , . * J C . '. (
(
6 6 ( ( J * J 1 ( (
6 6
( (* J ( ( :.(; ( . 6 (
:( (( * ( ( .
( 6 ( (* (
8
%A !
* (
6
(
y
T
T
R
m
m
θ
T
R
θ
mg
K K J ,
!
mg
x
"&
(*
(*
!
!
I .
I .
I I '= %
y
T
T α
R
(*
(*
!
!
T α
R
m
θ
m
θ
x
mg
mg
I . I J I . I * '= 6 (
**, * 1* S 2*
T3
T2
T1
T2
T1
m2
m1
m2 g
m1 g
m1 > m2
6 * ** I J 2
I I 1 I * (< * ; , * * * 2 * >
* 1 * < *
*
* 1
* - < / , I - R I . R 2 ,
>
* 1 * *2 2 0 **
I I !0
/ * 6 * 8
( (. ?(
%A B
(
6
(
S .
( B ( *' T
R
&
(
T
m1
m2
T
R
T
m1
θ
a
m1g
B ( *' x
a
y
&
(
m2g
m1g
θ
J J < K =
J ,
J J J < =
m2
m2g
*
( (. A
6 . *(
< = J < K =
&
J < K =
#
fs
µs N
F = fs
start to
move
F
6 (* .
( 6
(
+ 8 A
(( . ( 8
6 ( 8 ( 8
!"
6 ( 8
J
( 8 ( (
/ ( ( 6 ( @
/ A
( A
((
6
(
6 ((
J - $
. - ( Æ
( 6 ((
6
(
1 8
6
(
6 (
A
( 6
(
6 6 .
( (
( ( (
( ( . J - $ : - - 6 ( 6
f
fs
f
k
F
fs
%A
v
- J J - .. .
( - K nθ
si
mg
fk
θ
v=
0
1 ( ( ( ( *?( (
&
( Æ
( 6 ((
6
(
= 6 J - - % ( ( ( .
(
( ( (
nθ
g si
m
θ
fs
*= 6 % - ( ( .
.
!
6 % ( . .
- J J - , (
v
nθ
fk
si
mg
θ
& $ + .# . = y
T θ
y
T
T
11
00
00
11
00
11
R
θ
00
11
00
11
00
11
x
mg
x
mg
&
(
J J
&
(
J J,
< (
&
(
=
J J J ( : (
(. ( ( ( ( (
( (
( (
6 ( 6 (
J ( J
J ( #,
8 *
!. J !. ( J
*= / (
1 . ((
*( ( A
.
( 6 (
1 .
( *' ( .
v
B ( ( (
' ( . .
(( 6
.
J - $
<=
/ 6 (
( (
6 ( . 8
( &
?
(( (
radius
R
axis of fs
rotation
cylinder
wall
mg
$J
/ 6 <=
N
%
J 6 ( ( ( . ( .
6 .
= 1 *
8
8 8 / *
8
.
( (( $ J ( 6
(
8
( ?
((
6
J
B ( *
( N
J J - $ J - - - fs
6 ( *
( 6( ( .
O
R
mg
#
= 1 *
8
8 *' N
θ
y
x
O
R
fs
θ
&
(
$ &
(
θ
mg
J , $ J K <!=
<#=
J J : %8 6 ( 6
(
*(. ( *
( ( *
( ( 8 .
(( $J
S
$ J
$ K J
J ( J ( B &6
(
<!= <#= 8
K
( J
J % % ( <-=
mg tan θ
mv2
R
fs
cos θ
*( ( 6
(
( ( ( (
*( ( ( (
(
6
#!
( fs
cos θ
mg tan θ
/ (
( 6 ?
( ( ( ( ( 6
(
6 (
( ( (
mv2
R
+ .
2
% ( <B
(
6 ( ..=
( (( ( 6
(
6 ?
( 8
( &
(
( 6 ( ( A
((
6
(
6 <!=
K - $ $J
$J
- B <#=
$ K - $ J -
<
K - = J K - J - 1 % ( *
(.
( <B
(
6 ( .=
( (
( ((
6 (
( J * ((
( J ##
( *
. . - $ ( J ( 6 <!=
$
- $ J $ J K- B <#=
$ - $ J
<
- = J - J K - 1 ( *
.
K -
! 0 '1 # .
v
m
p = mv
:.(; ! . (( (
/ ( 6 6 ( ( 6 * ?
( ( (( A( 6 (
( *
F3
J /
6 ( 6 ( * J J 0
F2
m
F1
(
, ,
*(. (
( (6 B A
m1
v1
m2
v2
m’1
v1’= 0
Before collision
m’2
After collision
K J K #-
v2’
#
! 0 2.
.
(. *
(((
( 6 ( (. *
( A
6 < % 6= ( B ( (
(8 F
t
∆t
ti
/<
tf
/ J = /< =
/ J
J
J 0
/
?(
* * A
6 <= 6 (
(8 6 ( 0 (
# .
( ( '
( ( A
6 6 ( * ( .
' ( ( (( ( 6 ( (. *
*6 6( ( (. (
8
(
( / / *6 ( &
Fe1
Pi1
m1
F12
F21
Pi2
m2
Fe2
( ( &
6 (
6 (( 6 / A
A(
6 6 Before collision
1 (
&(
J #$
! 6 ( (( ( 6 ( (
J / K /
( (
(8 M
M/ J < K =M M/ J < K =M
. M/ ( ( 6 ( 16( ( 5 ( 6 /
/
J / K M/ J / K < K =M
J / K M/ J / K < K =M
16( ( (( ( 6 ( (
J / K / J / K / K < K = M K < K = M
J M J M MM J C
Fei
/ @ $ (
( (
6 5 J B (
( A
A( 6 ( 6 6 ( * J
J
m1
Fi1
Fi3
mi
m2
Fi2
( A( 6 *( :/ ( ( 6
( (( ( 6 ( (
m3
FiN
mN
8 6 J , (
J,
6 ( (( A( 6 (
( 6 (
@ ( (( (
6 ( ( 8
%C
! " !
1' & u1
y
m1
m2
u2
Before collision
v2
After collision
v1
x
m2
m1
J I J J I J 1'
B ** , *
* 1 , = ** *) E* *
1 * , * , = , @ * 2
, 1 2* 4 * 8(
*4 * * 9 * 2 * * 2 * * 2* * 2 *4
,) 2 * * 829 1 * *) * * * * * 1 * 1* * 2
1 * , = , * * 1 * , =
+
> 1 * 2 * 0 /1 1 3 * 4 *
1
* , = , * , 4* 1 * 4
3
J I . 3 I 8&9
#"
! ( (
6 ( 5( J 2
1
6 , J < K " =
. . 8 ( ( ( (
6 ( * ( ( *A . (8
(
K 2 J K .
( ( ( ( P 6 ( *A
/ 7( 6 ( 5( .
* ( 8 *( 8
( 8( <? <$=
(
(= /
1
!1
2 J K 6
< K " =
< K " =
B
6( ( ( * *A 8 8
( C
!1
2 J , 6 %
< K " =
:(
(( @ 8
( .
8 ( ( 6 6 ( ( .
( . ( A( 6 (
( (
( (( ( *A 5( 8 ( ( 6( (
1
< K " =
J K 1
( 8 ? ( ( ( ( ( ( ( ( ((
( /
( T (( ( ( ( 6 ( *A 6 ( * ( ( ( (
(
.
(
( *A T .( ( ( 5( (
<*
( ( 6 6 ( ( ( ( (=
"
# 8
( . 8 .
( * 6 ( (
:. . (
6 ( ( (
(
*
3 ( # y
m3
m1
( (
$ (
r1
r3
r2
m2
<=
<=
<=
x
rN
(
6 ( (
8
( 6 ( (
(
6 ( (
mN
5
(
( 6 J
. J K K K K K K J J < K J J < K J
#
K K =
K K =
-,
" #
C
J K K K J K K K ( (( 6 A
* <0=
J K . T (( ( 6 (
( 6 ( (
T (( A( 6 (
J K K K m1 F1i
< =
m2
Fi1
B <0=
mi
F2i
Fi2
K K K K FiN
mN
Fext,i
K< K K K K :(
J (
J K K =
=
K
=
K
J
K K K K<
K< K K K K < K K K FNi
K K J
=
( $ &(
( .
( A( 6 (
8
(
&
( 6 *(. 6 ( (
*8 6 ( ( (
(
A
6 6 %A
1N
CM
2N
G
6 J '
J L
K !L
#L9 J L
#L9
3N
J J # y
x
< (
6 ( 6 '. *( (
( ((
=
-
" #
%A
y
m1 at t0 + ∆ t
1
0
0
1
x y
(
A ( (
( (. 6 M
6( ( A
. 6
( < = B
( (
6 ( K M
( 0 , 0)
explode at t0
v0
φ0
11
00
00
11
00
11
m
projectile
motion
11
00
m2 at
00
11
00t0 +∆ t
11
x
0
(
6 ( A
( 6 ( ( 9( ( (
6 ( J L9 J J < ! =L
K < ! =L9
IL9
!
1( ( A
(( ( /6 8 (
.
(
<= <= ( ( A
88 ( 6 ( ;
(
.
6. ( *
:. J K M
K < K M= J L
K L9 J J < ! =L
K H< ! = < K M= J L
J ! K ! ! L9
-!
" #
3 ( # # + ,
%A
1 6 6 ( / ( 6 6 ( / 6 6 ( ( ( ((
y
y
111111111
000000000
R
000000000
111111111
000000000
111111111
R
000000000
111111111
000000000
111111111
000000000
111111111
000000000
111111111
000000000Ω
111111111
1111
0000
R
0000
1111
0000
1111
0000
1111
2
Ω2
1
1
x
x
y
1
111111111
000000000
R
000000000
111111111
000000000
111111111
000000000
111111111
000000000
111111111
000000000
111111111
000000000
111111111
000000000
111111111
2
Ω3
U (
*( &A
( * &A
( 6 6 J
J J
G
( ( 6 . 5
J,
J J
(
J
J
J
J K
=
J , <
x
-#
" #
3( 3 * ( ( 6 ( /
J . 3
C
J
J . 3
. 3< = J K %A
1 .
*( ( &
y
dφ
y
φ
−R
x
R
E(
*( &A
( ( * ( &A
( ( . ( 5
J !
6 ( (
J 3!
. 3 ( ( 6 ( .
J
J
3 !!
J
.3
!! !
J .
.
--
" #
%A
y
g
R1
1111
0000
0000
1111
0000
1111
0000
1111
R2
1 * .
( . .
( . (
5 / * ( *( ( *
( *' 6( +( (
5 ?
*
(
2 / 6 (
* ( *( x
(
&
(
A( 6
@ ( ( 6 ( (
6 , K < =
J
!
J ! ground
At final state
y
1111
0000
0000
1111
0000
1111
0000
1111
0000
1111
x0
16( ?
*
K J
!
J J ! x
ground
3 *. # # ( 6 $ (
8
(
8( /( ( 6 ( (
J
J /
( J
J
<0!=
J /
<0#=
-
" #
*
<0!= <0#=
J J ( 5 ( (( ( ( ( / . ( ( * 5
( ( 6 $ (
*8 6 ( ( 8
8
( (
8 6 ( ( ( ( ( *
6 (
(
J
J
/6
J
6 (( A( 6 @ (
J,
T 8(
6 ( 6 ( 6 (
O
%A
vCE
M
m
vmc
1 6
(
5
* / * 5
.
( 6 (
8 ( ( &
(
( A( 6
( 8 .
, J K (
J K , J K K J K
-$
" #
3 $
# , Time t
v
m
1( K M .
9( ( 6 ( system
being
studied
Time t +∆t
u
−∆ m
m+ ∆ m
1( (
(( (
v+∆v
system
being
studied
( 8
( 6 ( (
8 ( ( %(
C.8 ( * (
(( ( 8
( 8 (
8 ( (
= J <
1( (
K M (( (
K M= J < K M=< K M = K <M=
<
J K M K M K MM M
J K M K M< = K MM
C . 5
K M= <
= J M K M< = K MM
M J <
M J K < =
J M
J . J ( 8
( 6 ( (
(
8 ( ( :(
MM M J , M M , M ,
J J J K J J J -0
" #
%A
momentum conserved
u
mass = M
1
0
0
0
0
01
1
01
1
01
1
0
1
v
1 (
6
(
.
( 5
( ( 6 4 *&
( 6 *( momentum conserved
u
M
v
1 '( 9(
(
( 6 . 8
( 6 9(
*( (
8 ( ( %(
B *( J ,
J < =
<4=< = J 4< =
J
< = J < =
6 ( (
6 ( '(
/
*( ( (
( '( .
( 6 ( . 6 <
((= ( ( A( 6 (
( (
( '( *( ( (
( 8(
6 (
-"
" #
%A
'( ( * 9(
( (
6 .
( 8
( (
8 ( ( '(
+ve
M
g
dM
dt
J J <=
J vrel
%A
∆M
M
6
.( 8 ' &
( .
( ( 6 v
g
M
+
∆M
v+∆ v
+ve
J J <
=
J <, = J ,
J K
$
% &
z
y
r
P
x
/ * ( ((
6 * *( 5A A
+ *8 ( (
6 5A ( ( * / (
6 (
*( ( A
6 ((
y
P
r
φ
s
x
( # J !
#
!
J
(
-
H
( I
,
$ % &
y
P at t 2
φ2
P at t 1
φ1
x
3
' .( (
8 8
( 5 (( 8
( 5 * 5 ! ! M!
J
5 J
M
H
( I
M
! !
5 J J M
E
8 (( (
5 *
5 < = 5 < = M5
J M
J
H
( I
M
5 5
J J M
1 8
( 8(
z
z
w
y
y
P
P
x
x
w
( C E. 4 " +. E J 5
J 5 J K J ((
1( J , 5 J 5 J . 5 ( (
8
(
5 J 5 K $ % &
/6
!
J
5 K ! J 5 K K !
1( J , ! J ! J . ! ( (
(
5J
! J ! K 5 K 4
" , +. ,
y
∆s
r
∆φ
(
(8 M ( ((
8(
8 ( M!
6 M , M# J M# J M!
r
x
/ ( ((
8
( M# J !
J M
J 5
8 ((
(
8 *
J J 5
J B 8
( . '. 6 (
(
.
( &
(( ( (
( (. ( ( <(
( (
=
/ ( (
? (
C ( (( (
J J 5 J K !
$ % &
y
aT
a
aR
r
x
: B 6 (
J , J 6OO
'
% 5 %6.
5
(
y
1 6 . (
* ( ( /
(? *( ( ( ( 5 F
+ J P
r
. J (
x
y
F
θ
FT
+ J (
( 6 P
r
x
#
-
' % y
J (
( ( +
θ
P
r
F
x
FT
%A
Point
O
τ
θ
+ J + J 1 L
.
( (
(
.
r
mg
Point
O
τ
θ
r
mg
J 1 .
( (
(
(.
+
' % 5
" 2 ! "
E
(
y
F
1 (
( ( ( 6&A
* 6 ( B 8
( <
( ( (=
J J J θ
Fsinθ
m
r
x
z
(
J J 1 + J J : E*
( 6 ( 6 ( A
6 ((
+
5 , J 6 (
((
+
J ,
& :.( ! . 6 ((
( (
y
/. ' * &
( ( 6&A
' ( ( * P
m1
T1r
T1
l
T2r
r1
r2
z
T2
((
A
6&A
m2
x
A( 6
$
' % /( 6 /( 6 J K K J K /( (? ( ( *( 6&A
y
+
θ1
r1
K < = K<
T2r
h1
T1r
h2
J < = K < =
J < = K < = K< =
θ2
r2
=
< "" "" =
J < = K < = K < =
x
z
:(
((
J J <=
< = < = ( (
3( ( ( *(. * 7
J 7 S J 7 ( 6 (
( ? (
J 7 J 7 J <!=
E
(
&(
6 ? (
J
<#=
E*(
(( ? <!= <#= ( ? <= . *(
J 0
' % /( (?
J .
( A( 6 /? ( * ( 6 (
+ J + +
( (( 6 .
(
& J !
y
* ( (. &
( ( ((
(
Σ F1T
m1
J
radial
direction
m2
r2
x
Σ F2T
r1
= K < =
= K < =
<
+ J < radial
direction
τz
( (
*(
( (? +
J < = K < =
J < K =
. ((
(
6 ( (
6 (
( *( ( 6 A
+ J ,
. , J K 6 $ &(
*
y
( ( ( 6 (
5
m1
r1
m2
r2
rN
,J
x
mN
"
' % / (( (? (
(
(
+
J
J ,
: * ( (
.
(
( * 8 5A (
8 (
.
(
( ( ( 6 ' * %A
y
30
θ
m2
J !# '
J #! '
J '
o
5
3
θ
4
m1
x
m3
<= B
( 6 (
6 ( ( *( A
( (
8
<*= 6 - : 6 ( . ( ( 6 ( (( *( (
A
( ( ( +( ( (
2
1.
<= 5
(
,
J
J <!# '=<, = K <#! '=<# = K < '=<- =
J # ' E
, J <!# '=<# =
, J <!# '=<- =
K <#! '=<, = K < '=< = J " ' K <#! '=< = K < '=<, = J 0 ' Chapter 8 Rotational Dynamics
48
θ = sin−1 (3/5) ⇒ θ = 37◦
(b)
∴ τz = 4.5 N × 5 m × sin(30◦ + 37◦ ) = 20.7 N m
But
τz = Iαz
∴ αz = τz /I3 = 0.18 rad s−2
8.3
in clockwise direction
Parallel axis theorem
C.M.
axis
z
Iz = ICM + Mh2
h
Iz = Moment of inertia rotating about z-axis,
ICM = Moment of inertia rotating about the axis
passing through C. M.,
C.M.
z-axis is parallel to the C. M. axis and h is the
distance between the two parallel axes.
M
Proof
z’
z
slab // to
z & z’ axis
mass mn &
coordinate
(xn , yn )
h
For the Iz about the z-axis:
mi ri2 =
mi (x2i + yi2)
Iz =
C.M.
i
i
Let (xCM , yCM ) be the x, y coordinates of the
C. M. measured from the x, y coordinate sysy’
y
z
rn
(xn , yn )
(xCM, yCM)
h
x
tem.
xi = xi + xCM
yi = yi + yCM
x’
' % , J
J
J
H< K = K < K = I
< K ! K K K ! K =
< K = K! K! K < K = $,
!
¼ J , K ¼
: / A
( ( ( A
(( ( ( ( 6
(
( ( A
5 " # ,
,J
( 6
,J
+( 5
( (2
p
∆q
f(qi )
p=f(q)
q
qinitial
"
"
q final
qi
<8 =8 J
<8 =M8
$
' % %A
axis through C.M.
at the middle
G
6 .
( ( 1
(
(
( . ( ( .
(
( M
( ( ( ∆x
x
xi
M J 9M 9 J ( <
' =
+L/2
−L/2
, J M J
( 9 J "1
#$
#$
J
#$
#$
9
1 1 J #1 - J ! 1
, J H I
#
#$
#$
A
(
L/2
L/2
C. M.
z
,
J , K J ! 1 K 1!
J # 1
%A
z
z’
x
11111111
00000000
dx
00000000
11111111
00000000
11111111
00000000
11111111
00000000
b 11111111
00000000
11111111
00000000
11111111
a
00000000
11111111
1 6 ( ( ((
*( A
( ( (
(
(
( ( ( (
.
( .
( A
( 6 ( (
J <=:
: J (
( 6 (
( ( (
*( @;
, J J : !
!
( 6 (
6 ( (
*( 6
, J , K J : K : !
$!
' % ( : J "<=
, J
! K J ! K /( ( 6 (
*( 6 &A
K , J
, J
!
J ! <= K # H I
$
$
$
$
$
$
K J !
!
J ! < K =
%A
z
1 6 ((
*( ( (
( ( ( R
J <=9
9 J ( <' =
C ( ( 6 (
8 *
,
J
y
dθ
dm
θ
J
J
%
R
x
J !. !.
J %A
1 6 ' ((
*( ( (
9
!
.
$#
' % y
1111111
0000000
0000000
1111111
z
0000000
1111111
0000000
1111111
0000000
1111111
0000000
1111111
0000000
1111111
ring with
radius r and
thickness dr
x
R
( .
( (
' . J
H.< K = . I
6 ( <' =
H.
J .
K!.I
.
!.
J ! ( 6 (
6 ( ((
*( ( (
! , J <= J
/( ( 6 (
6 ( ' *( ( (
! ! J
J , J , J
- !
5& /6.,. # + ,
B * ( ( ( ?
*
( 6.
(
( * (
5
= J ,
!=
+
J , *( 6 6 (
$-
' % V(
6 . '. J , + J , *( (
( ( . ( (( (? *( ( 6 (2
z
6 ( ( (
ri −rP
+ J + K + K +
P
J < = K < = K K < =
ri
rP
x
J ,
O
:. . ( (? *( ( (
Rigid body
y
+ J < = K < = K K < = J H< = K < = K K < =I H < K K K =I
8 (
J ,
J ,
6 J , <
((
?
*
(*
= + J , *( 8
( ( + J , *( ( ( ?
*
( * (*
Fi
&
&
&
&
&
%A
1 ( * ?
*
J,
L
L
4
M
K K K J (
K K < K = J (
m
y
R1
rR2
rR1
R2
z
x
rM
Mg
mg
/' . (
8
K < K = J ,
<-=
/' ( *( (
+ J K < = K + J 1! K 1- K 1! J ,
$
' % /6
<-= K <=
! J ,
<=
! J < K = K ! J K #! #
J K !
J J K !
!
-
%A
frictionless wall
a
2
a
3
/ *(
?
*
J ,
M
h
C.M.
rough
ladder mass = m
O
a
R1
f
mg
O
J
J < K =
B
(? *( ( A
(
( ( ( K J J J K R2
Mg
$$
' % %A
α
6 * 6 3( ( (
( ( (
6 K J , <$=
J ,
<0=
/' ( (? *( ( A
( (
( ( 1 < K = 1 J ,
< K "!= J
< K =
θ
L
M
#
y
T
α
θ
Fv
B <$=
"!= J < K = < K
<
K =
B <0=
"!= J < K
<
K =
Mg
mg
O
x
Fh
'
'
50 6.,. . . %A
M
R
T
y
T
m
mg
B
(
.
( J J /? (
+ J J ,
. , J 6 ' ((
*( ( (
J !
! ! J <"=
$0
' % ( 6 ( ( ( .
(( J <"= *
J ! ! J C
!
J
K !
!
J
J
K !
J
%A
B
(
.
( +ve
T2
T1
m
mg
<=
<,=
/( (? R
T1
J ! J !
T2
2m
2mg
+
J J < =
J <=
<!=
( <= <,= ( <=
<! != < K = J ! <#=
E*(
(( <!= ( <#= . *(
! !<= <= J ! J ! K #
J "!K # J J "!K #
$"
' % 53 6.,. . 6 J , + J , *( A
( (
6 ( * *( 6&
((
( (
6 ( B ( ( . 6 ((
= 1A
6 ((
( *= ((
A
. ( (
%A
mass = M
.
( N
α
J J $
R
f
'
Mgsinθ
/( (? ( + J J , J !
J ! aC.M.
Mgcosθ
θ
( <= ( <-=
!
<-=
<=
J ( J (
'
'
'
!
'
'
'
'
J '
'
J !# J J #! '
'
%A
ωo
'
1 6 6 8
(
8
( 5 ( . 6 &
@( 6 / Æ
( 6 '
(
6
(
*(.
( ( 6 - (
( ( 8 ( 6 *( 6( (
.
(( *
$
' % <= +( ( 8
( ( (
2
<*= +( ( 8 6 2
E(
<= ( (8 , N
α
J - $ J - <$=
(( .
(
(
+ '. ((
9( ( J , 8
( 6 ? ( @
ωo
aCM
J J J
f
Mg
<0=
<$= <0= 8
- J - J , J ! J !
J <"=
<=
3( 5 * ( 8
( ( . 5 (( '.
(
5 J 5 K J 5 5
1( (
J 5 J
( <= ( <!,= . 8
5 "
! J 5 "
! J 5 <!,=
<!=
0,
' % ( <$= ( <!= . 8
!- J 5 !- J 5 ( <"= ( <!!= . 8
!- - J 5 J # 5
<!!=
<*= B <"=
J
-
<!#=
J #5- %A
T
α
R
Ro
a
total mass
=M
Mg
B /. (
' (( (
.
( .
( ( 1 ( (
* 8 ( (
( J + J J < =
<!-=
+ J , J <!=
!
<!-= <!= 8
< = J !
J !< = <!$=
J E*(
((
<!0= ( <!$= . *(
J !< = J !K!
<!0=
0
' % C
J
J !K!
(
) 7 '1
1 ( 6 ( *( ( ( 8 *
z
7 J J / 7 J / 7
/
J
<
/= J
/ K /
J <= K y
7
/
J
J J +
. ( (( 6 (
:( (( *( 7 + ( *8 ?(
(
l
O
v
θ
r
x
* 5 .
( ( ( ( %A
y
b
P
O
θ
r
m
111
000
000
111
000
111
θ
F=mg
x
1 (
6 6 ( ( (
/? .
( ( ( (
+ J J <
.=
8
7 J <
.=
J 0!
0#
( ) 1 + J 7
J
<
= J J
B ( 6 ( 6 (
8
( 6 7 7 7 /( ( 6 ( (
1 J
1
7
<* 5
(
=
7 J +
J
<+ J + K + =
E(( .
(( 6 ( (? :/ (
*( ( 6 1 1
J
+
/ 6.
5 . ( *(. (
Linear momentum
p ∆ p//
F//
F// =
F
Angular momentum
τ//
∆ p//
∆t
L ∆L//
τ// = ∆L//
∆t
p +∆ p
∆p
τ
L +∆L
p
F =
∆p
∆t
∆L
L
τ = ∆L
∆t
0-
( ) 1 ((
*8(
∆L
L +∆L
+ J < =
<
(
.=
M1
+ J
M
L
M1 .
* (
.O
1 1 ( (
( A
( A
.
8 .
C.M.
r
Mg
7
+. . +. z
ω
θ
11
r’ 00
00
11
00 p
11
θ
O
x
r
y
1 (
(
:. ('
( 6 ( (
7 J / ( ( 5
( ' ( ( <
/ S = ( 7
8
( 5 ( G .( (
.
( 7 5
8( 2
0
( ) (. ? 9
( * ( (
7 "" 5
7 ( 5 6 ( * (
*( ( ((
A
1 7 J ,5 < . 8 ( &
(
/ J 6 (
= +(
( (
*(. 7 52
B 8
5 6 ( &
(
z
=
2
1+ 2
p 11
2 00
00
11
11 ω
00
1
11
00
00
r’ 11
00
11
00 p
11
1
r2
r1
7
y
O
x
J 7 J / J <= J <5= < J 5=
( J 7 J 5 J ,5
:(
/ 6&( 6 ( ? ( ,5 / (
( *(
6 (
( ( 6 *
6 ( * (
*( ( ((
A
1 J ,5 B 1
5 *( ( ( ((
6&A
1 J ,5
6 + J + L& K + L' K + L
+
J 1
/( ( 6 , (( + J , 5 .
( * O
0$
( ) %A
/( ( 6 ( (
1
M
R
x
O
K
<(' (
(. ( * K8=
J ! 5 K /( A( (?
y
rm ,
5
1 J ,
J 7 K 7
m = mvR
+
m
J + J + J
p= mv
1
! 5 K J ! K J
mg
. ( (
6 ( ( (
6 ( ( J "
J K !
!
J K!
7 (
# +. *.
1 . '.
+ J
1
6 ( (( A( (? (
( ( @ (
( 8
#
J , /
00
( ) %A
=
Ii
If
, %,
J, 5
1 8
( ,5
wi
!=
Lw
wf
−Lw
Ls
(
. &
(
1 5 (( (&(*
(
1
(
stationary
turn table
1
J 1
(
1
J
J
J
1
1
1
K <1 =
1
(
(
1 J !1
(
7 $, # + ,)
τ//
Li
+ 6 (
6
M
M1 J + M
$$
Lf
∆L
Object is symmetric about the rotating axis
$$
E
*(
( ( ((
0"
( ) τ
Lf
θ
∆L
Li
Object is symmetric about the rotating axis
+ 6 (
6
M
M1 J +M
E
((
( J M1 J +M
1
1
6 1 6 5A +M /( ( ,
8 (*
( ((
%A
= 1 *
/ . ' ( (*
(
= E
(
(
/ (
z
z
dφ
Lsinθ
L
CM
r
Mg
O
x
L
dL
τ = r Mg
L + dL
θ
y
O
y
x
(
( 1 ( ( ((
A
<
5=
%A( (? ( ( + J 8 (
(8 M M1 J + M J M 0
( ) 8
M1 J <1 =M!
M
M! J 1 M
J 1
:(
(( ( A( (? ( (
6 1 *( ( ( ( 6
1 < + 1 1 1 =
/ 8( 1 < ( ((
A
= 8 . *( ( 8(
A
< &
=
18 6 5
J MM! J 1
*+, & -) ! -)
89 , #
( (
(8 6 M A
(( 6 (
(
(8 ( ( 6 M +' * ( 6 ( y
∆S
111
000
000
111
000
111
θ
F
* J M J
x
+' * ( (
8 &
(
8
. 5 *
*
J
M +ve work done
∆S
11
00
00
11
00
11
00
11
∆S’
F
",
−ve work done
11
00
00
11
00
11
00
11
F
"
*+, ! & -) ! -)
89 , , #
E ( (
( 6 <=
F
∆x
8
( . ( 6
( ( $ (
(
.
( &
(
M
F(xi )
F(x)
( &&( (
(
(
8 (&
8 A
( (( (
< =
x0
x
xN
xi
+' (
(
(8
M* < = J < =M
xi+1
positive
negative
work done work done
* J M* J
<= J *
/( .' 6 ( ( 6 ( < =M J
<=
( ? ( ( (( 6 ( 5 .
( (
8 6 (
8 <= (
8 6 (
8 <=
"!
*+, ! & -) ! -)
%A
x
E
6
m
F = −kx
* )
J
J
equilibrium position
x=0
<=
J ! < =
)
F(x)
x
F = −kx
%A
y (−ve)
y (+ve)
F = −mg−ky
y=0
equilibrium
position
y
F(y)
−mg
m
( (
6 * )
J J
<=
J
< =
J < = ! < =
)
)
"#
*+, ! & -) ! -)
y
/9( 6 (
8 *
<= J <=L& K <=L'
F(r(t))
r(t) ∆r
r(t)
B ( ( 8 *
<= J <=L& K <=L'
r(t + ∆ t)
x
(
8
6 <= ( < K M= ( (
(8 M
6 M , 6 A
* (
(
(
(8 (( <<==
+' (
(
(8 .
( ( M
M* J <<== M
< ( ((
6 ( (
*(OO=
*
J
%A
y
φ
L
T
φm
φ
F
F
x
mg
1 * (
.
( ( 1 (
1 6 .
.
@( ( 6( ( ( ! ( ( 8 .
( (( (( ( (
( 6 * ( B
(
.' * ( 6 "-
*+, ! & -) ! -)
1.
6 (
( 6 @ J , J ,
! J , ! J ,
J ( !
( ( M 6 ! ! K M!
y
M* J M J M
*( J M
(
∆φ
φ
J 1 ! !
M* J ( ! 1 ! ! J 1 ! !
C
∆S
x
x
J 1 !
*
x+∆ x
J
*
1 ! ! J 1< !
=
89+ vi
11
00
00
11
00
11
O x
i
00Fx
11
11
00
00
11
00
11
(
6 ( (
( ( &( 6
( ( 6 (
vf
11
00
00
11
00
11
x
xf
J J
J
+' ( * ( 6
*
J
J
J
*
< =
!
* J
5 '
(
; J ! J ; ; J M;
<
? <==
<=
"
*+, ! & -) ! -)
6 + (
8 % M; % ,
6 + (
8 M; ,
: (
6 8
(
8 (
( *( 8 6 '
(
( ( *( ( ( * J M; (
6
89 9 + y
F
φ
dS
dθ
r P
* 8
( *( ( ((
6&A
.
( 6 (
( +' * ( 6
* J < != J ! J + . + ( 6&( 6 ( (? *( (
x
6 ( * ( 6 ( *
6 ( (? ((
J
*
.
w
+ J+ J *
J
+ J + 5
( 6 ( * (
6 (
& 8 *
; J J < 5 = J 5
!
!
!
/( ((
'
(
6 ( *
; J 5
; J
!
v2
r2
m2
r1
m1
v1
J !
5
"$
*+, ! & -) ! -)
!
, ( ( 6 (
6 ( * *( ( ((
A
; J ,5
& : + B (
M; J , ; J ; B (
M; , ; ; B ( (
( (. *9( (
' (( 6( %A
(
. ( 5 *.
m1
m2
u
16( K K B <!=
J
E*(
(( <-= ( <#= . (
J J K J K K ! J < K = ! K < = J ,
E8 6 ( . 5
! J
J
K K <!=
<#=
<-=
"0
*+, ! & -) ! -)
0 (
#
((
5 6 8(
8 6 .
(
8
( 6 P (( %A 6 8(
8 6
= E
!= 8
((
6
#= * 6
%A 6 &8(
8 6 & 6
(
V(
1 5
(
6 8(
8 62
5
(
1 8(
8 6 6 (( 6 (
8 ( P 6 (
6 ( .' * ( 6 ( (
6 *
( ( (
( *
( ( . * ( *
(
(
:( ((
= <= 8 6 6 ( A
( 6(
!<= ((
!<= J <=
!=
J ,
+' 6 ( <
((
( ( (= @
6
y
1
B
1
A
2
x
O
x
A
B
2
Chapter 11 Work, and Kinetic Energy and Potential Energy
88
F · dr =
path1
F · dr
path2
∴ Travelling from point A to B, then back to A, the work done is:
WA→B→A = WA→B + WB→A =
F · dr + −
F · dr = 0
path1
11.7
path2
Potential Energy
Consider a particle moves in the influence of a conservative force, which is position dependent, i. e. F (x). Now the particle displaces from xi to xf , potential difference ΔU is
defined:
ΔU = Uf − Ui = −W
where W is the work done by the force during the displacement xi to xf .
Or
ΔU = U(xf ) − U(xi ) = −
xf
F (x)dx
xi
def
If for a particle reference point x0 , the potential energy is defined as zero, i. e. U(x0 ) = 0.
x
U(x) = −
F (x)dx
x0
In particular,
x
U(x) − U(0) = −
F (x)dx
x
d
d
[U(x) − U(0)] = −
∴
F (x)dx
dx
dx 0
0
⇒
dU
= −F (x)
dx
"
*+, ! & -) ! -)
E
J /' ( ?
*
(
( * J , ((
< <,= J ,
F = −kx
x
< <= < <,= J m
< <= J <=
<
=
< <= J ! equilibrium position
x = 0, U = 0
/
<
J ! <!= J J B 6 8
(
/' < <,= J ,
y
< < = < <,= J <=
< <= J <=
< <= J y
F = −mg
/
y = 0, U = 0
<
J J 4 (
# * /+
M< J < < J *
Ui
initial
position
vi
<=
Uf
final
position
vf
,
*+, ! & -) ! -)
( * J <= ( .' * ( 6 ( 9 6 B 8
(
*J
<= J ! < = J ; ; J M;
<$=
E*(
(( <$= ( <= . 8
< J; ;
< K; J< K;
M< J M;
<
(
( . 8(
8 6 A
( 6 (
8
8
(
*
((
((
(
8
(
. 8 6 ((
<
8( 6
6 *= ((
<*( 5A A
= (
:. . ( ( (( *( ( 8
( * ((
* ( 6 (
/( % 6 ( *
<0=
;J
!
:( ((
y
mn
rn’
rn
CM
rCM
x
J K J K . J 8
( 6 & .
( ( ( ( %(; 6
J 8
( 6 ( *; ( 6 .
( ( ( ( %(; 6
J 8
( 6 & .
( ( ( ( *; ( 6 B <0= . *(
< K = < K = J < K ! K =
;J
!
!
( ( (
< = J
< = J *+, ! & -) ! -)
1 J < =" < = J J ,
1 ( (
(
J < 5= J ,5
!
!
!
. 5 ( 8
( *( A
( ( ( 6 ; J K ,5
!
!
( ( /(
( 6 ( 6 ( ((
! ( ((
( .
( ((
*( ( A
( ( 6
( ((
A
( 8
5 U(x)
E4
E3
K(xf )
K(xg )
E2
E1
U(xf )
U(xg )
E0
xa
xb xc xd xe
xf
xg
x
(
A
8(
8 6 5 .
( ((
< <=
1( J +
J ,
<= J <
*+, ! & -) ! -)
!
J (* ?
*
& ( (
A
(&
6
J
(* ?
*
& (
A
6 (
(
(
+
J
( ?
*
& (
A
6
< <= K ! J = . = ( 8 (( %A
6 = J = . ( 8
5
= J ; < = K < < = ( J = J ; < = K < < = ( J +
+
+
6 ( 6 ( (
= 7( ( .
8 7( *8
6.
= 6 = J = (
( ((
( J != 6 = J = (
( ( J #= 6 = J = (
( ( (. 8 C.8 6 ( 6 (
8 ( ( 8 ( ( ( ( 8
-= 6 = J = (
( ( % = 6 = = (
* .
6 < <= '. ( * ( .' ( ( (
(
%A
6 ( J , < J ,= J < J ,= J , E < <= J = J H<,=I K H <,=I J J ((
!
!
!
#
*+, ! & -) ! -)
1( (
J !
! ! K ! J ! . J <= J <=
J J < <= K J
/ 8
J ( J J J J J J J
6 J K 6 J .
J ! (
J J J ! (
.
.
!
J ! J .! !
.! K .! J .
.
. K ! J J . -)
6 A( 6 (
( ( ( @ ( 8(
6 *
M; K M< J *
. * ( .' ( ( * ( A( 6
%A
Uspring
+K
Wspring
Wspring
K
Wgrav
Wgrav
K + Ugrav
K + Ugrav
+Uspring
Earth
Earth
Earth
Earth
E( J M; J
* K *
E( J K
E
M; K M< J
E( J K
%(
M; K M< J
*
*
E( J K
%( K E
M; K M< K
M< J ,
2 + # M; K M< K M= J *
-
. -)
. = ( ( 6 ( (
( ( % ( .
( ( (
6 ( < ( ( ( *9( ((= ( % ( .
( 6 *(.
(
= J ; K <
3'
( ( 6 *
J 6 *
:(
(( ( (
( ( 6 (
6 ( ( &
* Fext
J < (
( 8
?(
* : :(
(( (
( ( .' ( 5
(
T 6 ( ( (
( * =
dxCM
CM
CM
J J J ( 6 ( ( 8
( 6 ( J J ; ;
!
!
< ; J =
# J M; 6 ((
T ( 6 <= ?(
. # ( ( 6 ( ( 6 M; K M< K = J *
T 8(
6 <%= ?(
WWW/ ?(
( ( .'& ( 6 (
# ( (
6 ( *( ( ( ( 6 ( ( (( ( 6 ( Chapter 12 Conservation of Energy
12.2
96
Some examples of conservation of energy
1) A sliding block is stopped on a horizontal table with friction.
Center of mass (COM) energy equation:
2
−f sCM = − 12 MvCM
Conservation of energy (COE) equation:
2
Wf = − 12 MvCM
+ ΔEint,block
2) Pushing a stick on a horiozntal frictionless table.
Center of mass (COM) energy equation:
Fext sCM
Conservation of energy (COE) equation:
1
1
2
Fext s = MvCM
+ Iω 2
2
2
Fext
S
1
2
= MvCM
2
SCM
CM
CM
If Fext is acted on center of mass,
s = sCM
1
2
Fext s = Fext sCM = MvCM
2
3) Ball rolling down an inclined plane without slipping
Center of mass (COM) energy equation:
1
2
(Mg sin θ − f )sCM = MvCM
2
Conservation of energy (COE) equation:
1
1
2
+ Iω 2
Mg sCM sin θ = MvCM
2
2
Mg
SCM
f
Mg
θ
acts on CM
Notice that the frictional force does no work in the COE eq. as the instantaneous point
of contact between the ball and the plane does not move.
Chapter 12 Conservation of Energy
97
Example
Two men are pushing each other. m2 is pushed away from m1 by straightening their arms
and the force between them is F .
(a) What is the speed of m2 just after losing contact?
(b) What is the change in internal energies for m1 and m2 ?
m1
m2 is pushed
to move forward
m2
frictionless floor
Answer:
(a) Consider m2 as one system, COM eq. is:
1
2
F sCM = ΔKCM = m2 vCM,m
2
2
where sCM is the displacement of the center of mass of m2 .
2F sCM
∴ vCM,m2 =
m2
(b) For m2 , COE equation is
where
ΔK + ΔEint,m2 = Wext
ΔK = ΔKCM = |F sCM |
Wext = |F s|
.
Note that s is the total extension of m1 ’s hand (i.e. the displacement of m2 ’s hand
when a force F is acting on it, where s = sCM ).
∴ ΔEint,m2 = |F s| − |F scm |
For m1 , COE equation is
ΔEint,m1 = Wext = −|F s| (F opposite to s)
/.
! # -
;
F21
m1
m2
r21
(
6 (
8 ( 6 A
* ( (
6 (
8 ( 6 A
* ( J > L F12
m1
r12
m2
J > L
; /
.#
6 . ( %( ( * ((
6 m
J 6 ( %(
J 6 ( %(
8
((
( ,
,
RE
ME
>
J >
J
,
,
"
,
,
/.
/< # /
"
Fc
T
w
T
Fc
α
mgo
mgo
φ
φ
* (
( (
8
( 6 ( ( 8(
J K
3.
J K < = ! !
:( (( J # . # J 7(
8 8
(
B ( ( ( ?( ! J ,
J K < = ! J # J 5 # <! J ,= J 5 ,
,
3( * ( *(. ( (
( 8(
A
G
E
3. ( * ( ( *(. ( (
( 6&A
J <. !=
5
J < K !=
,,
/.
5 J ! K ( !
5 J ( ! K ( !
!
( J 5 5 !
; # . .# / 1 6 ((( A( (
6 ( 6 ( .
(( ( ( (
/ !
1 6 A( 6 (
( ( & ; +
ra
m
M
11
00
00
11
00
11
00
11
a dr
F
rb
M< J < < J * 6 ( *
J
J
J > >
b
/.
,
I J> 3 % * ,M 3 * % ,
9(
(( * ( .' * ( 7
((
3 *
3 ( *
( 3 ( L< I < < I * I > 9. . (' I < I < ;< . < ;< I ,
< ;< < ;< I > < ;< I >
I*
% A (
7
(
3 5@ ( 3 3 ( %( 2 ( (
( 3 ( %(: 7
((
3 5 ( (
* 3 ( ( (7
( 5
(
> J I,
!
,
0 + # m2
r12
m1
<
I
r23
r13
> m3
J > J > % >
( (' ( ( (
( ( 5
(
= I <
,!
/.
3 /+ # (
( *
(
(
M
ω
r
m
J >
; J J <5= J 5 !
!
!
<
6 ( 8
((
6 8
( (
( 6
>
J 5 >
J5 >
;J
! > > J >
= J; K< J
! !
Chapter 14
THERMODYNAMICS
14.1
Introduction
Thermodynamics is the science of energy & energy-conversion.
•
Macroscopic description of matter
State variables, temperature and the zeroth law of thermodynamics, phase change,
ideal gas processes
•
Heat, and the First law of thermodynamics
Heat as energy transfer, heat & work for ideal gas processes, 1st law of
thermodynamics, thermal property of matter
•
From MICRO to MACRO, entropy, and the 2nd law of thermodynamics
Molecular properties of gases, thermal energy and specific heat, the concept of
entropy, 2nd law of thermodynamics
•
Heat engine & refrigerator
Heat to work and vice versa, ideal gas engines, the Carnot cycle, limit of efficiency
(perfect vs. real engine)
Chapter 14 Thermodynamics
14.2
104
Macroscopic Description of Matter
Thermodynamics deals with MACROSCOPIC systems, rather than the “particles”. It is all
about energy and energy conversion, especially that of converting HEAT ENERGY into
MECHANICAL WORK. (So, the word thermo-dynamics)
14.2.1.
State variables
The set of parameters used to characterize or describe the “state” of a macro-system.
e.g., mass, volume, pressure, temperature, thermal energy, entropy… (are not all independent,
however)
Change of state variable: ∆x = x f − xi
•
A system is in THERMAL EQUILIBRIUM if the state variables stay constant with
time. Two or more systems are in thermal equilibrium with each other when their
respective variables are unchanged upon making thermal constant.
•
If system A and B are each in thermal equilibrium with a third system, then A and B
are in thermal equilibrium with each other. (zeroth law)
•
Mass density: ρ = M
V
,
Number density: N
V
m(12 C ) = 12 µ
Atomic/Molecular mass: m(1 H ) = 1.0078µ ≈ 1µ , µ --atomic mass unit
m(O2 ) ≈ 32µ
Moles and Molar Mass: 1 mol ≈ 6.02 × 1023 basic particles
N A = 6.02 ×1023 mol −1 --Avogadro’s number
The number of moles in a substance containing N basic particles is n = N
NA
.
Chapter 14 Thermodynamics
•
105
The number of atoms in a system of mass M (in kg) is found by N =
M
, m is the
m
atomic mass.
•
The molar mass is the mass in grams of 1 mol. of substance. M mol (12 C ) = 12 g
M mol (O2 ) ≈ 12 g
•
mol
mol
,
.
For a system of mass M consisting of atoms/molecules with molar mass M mol , the
number of moles of the atoms/molecules in system is n =
M
.
M mol
Example
The 12C atoms weigh 12 g by definition, so the mass of one 12C atom is
m(12 C ) = 12 g
NA
= 1.993 × 10−26 kg .
On the other hand, we also defined that m(12 C ) = 12 µ , so 1µ =
m(12 C )
= 1.661× 10−27 kg .
12
For any other substance, one way to find its atomic mass is, e.g.
m(O2 ) = 32µ = 32 × 1.661× 10−27 kg = 5.315 × 10−26 kg
14.2.2. Temperature
A measure of system’s THERMAL ENERGY, the kinetic and potential energy of
atoms/molecules in a system as they vibrate and/or move around.
Two systems that are in thermal equilibrium have the same temperature. In other words, the
temperature of a system is a property that determines whether or not a system is in thermal
equilibrium with other systems!
•
Temperatures scales
1. Kelvin scale ( K): Ttr = 273.16 K , T ( K ) ≥ 0
Chapter 14 Thermodynamics
106
9
2. Celsius and Fahrenheit: Tc = T − 273.15 , TF = Tc + 32
5
The temperature in Kelvin scale is adopted as fundamental in physics! It is sometimes
called the absolute temperature scale. At the absolute zero temperature ( T = 0 K ),
Eth = 0 .
•
Measuring the temperature – thermometers
Use the properties of a substance that vary with temperature. For example the pressure
of a gas at constant volume, the electrical resistance of a wire, the length of a metal
strip, the color of a lamp filament, etc.
Let X be a parameter property that is linearly dependent on T , T * = αX . At the
triple point of water, 273.16 K = α X tr , from which, α = 273.16
at any other temperature, T * = (273.16 K )
X tr
is found. Then
X
.
X tr
The pressure in a constant-volume gas thermometer extrapolates to zero at
T0 = −273o C . This is the basis for the concept of absolute zero.
14.2.3. Phase changes, phase diagrams
A substance may change phase, e.g. from solid to liquid by heating. For example, water
solidify (freeze) at the freezing point, but vaporize (boil) at the boiling point.
At the freezing (melting) point, the solid phase (ice) and the liquid phase (water) are in Phase
Chapter 14 Thermodynamics
107
equilibrium, meaning that any amount of solid can coexist with any amount of liquid.
Similarly, at the boiling (condensation) point, the liquid and vapor phases of water are in
phase equilibrium.
Note that only at those boiling and melting points that phase equilibrium can be maintained!
•
A phase diagram is a diagram showing how the phases & phase changes of a
substance vary with both temperature and pressure.
Examples
The following figures show the phase diagrams of water & CO2 . Three phases of matter are
the solid, liquid and gas.
Chapter 14 Thermodynamics
108
The right figure below shows the temperature as a function of time as water is transformed
from solid to liquid to gas.
14.2.4. Ideal Gas Processes
•
Ideal Gas
The potential-energy diagram for the interaction of two atoms is shown in figure. Solid and
liquid are systems where the atomic separation is close to req . A gas is a system where the
average spacing of atoms is much greater than req , so atoms are usually not interacting.
Chapter 14 Thermodynamics
•
109
An idealized hard-sphere model of the interaction potential energy of two atoms
A gas of atoms obeying such an interacting potential is called Ideal Gas. The ideal gas model
can be good approximation of a real gas when its density is low and its temperature is well
above the condensation point.
•
Molecular speed
Atoms in a gas are in random motion at T > 0 K . The distribution of speed is
outlined as follows.
(a) The most probable speed vP =
(b) The average speed vav =
2kT
m
8kT
πm
(c) The root-mean-square speed vrms =
3kT
m
Chapter 14 Thermodynamics
110
A histogram showing the distribution of speeds in a beam of N 2 molecules at T = 20o C .
•
The Ideal Gas Law and Ideal Gas Processes
The ideal gas law (thermal equilibrium): pV = nRT = Nk BT
Universal gas constant: R = 8.31 J
Boltzmann's constant: k B =
In a sealed container, we have
gas is given by
mol ⋅ K
R
= 1.38 × 10−23 J
K
NA
pV
= nR = constant, and the number density of atoms in a
T
N
p
.
=
V
k BT
An ideal gas process is the means by which the gas changes from one state to another. The
p-V diagram is a graph of PRESSURE against VOLUME. A point on the p-V diagram
represents a unique state of a (sealed) gas.
Chapter 14 Thermodynamics
111
A quasi-static process is one that when the system changes state from, say 1 to 2, it is done so
slowly that the system remains (approximately) at thermal equilibrium. Thus, a quasi-static
process is reversible.
V f = Vi
(a)
Constant-Volume (ISOCHORIC) process:
(b)
Constant-Pressure (ISOBARIC) process: Pf = Pi
(c)
Constant-Temperature (ISOTHERMAL) process: T f = Ti
(d)
ADIABATIC (no heat transfer) process: Q = 0
Example
A gas at 2.0 atm pressure and a temperature of 200o C is first expanded isothermally until
its volume has doubled. It then undergoes an isobaric compression until its original volume is
restored. Find the final temperature and pressure.
Chapter 14 Thermodynamics
112
Solutions:
For process 1 → 2 , T2 = T1 = cons tan t . Hence, we have
P2V2 = P1V1 , or. P2 =
V1
P
P1 = 1 = 1.0atm
V2
2
For process 2 → 3 , P3 = P2 = 1.0atm .
Since
V3 V2
=
, we have
T3 T2
T3 =
V3
V
1
1
T2 = 1 T1 = T2 = × (200 + 273.16) = 236.5 K = −36.5o C .
V2
2V1
2
2
Chapter 15
Heat, the First Law of Thermodynamics
15.1
•
Work and Heat
Work is the energy transferred to or from a system due to force acting on it over a
distance.
•
Heat is the energy that flows between a system and its environment due to a
temperature difference between them.
•
Energy conservation says: ∆Esys = ∆Emech + ∆Eth = Wext + Q , (Note: not ∆W &
∆Q !!) where E sys = E mech + Eth is the total energy of the system
E mech = K + U is the mechanical energy associated with the motion of the system as a whole
(macroscopic E ), K is kinetic energy and U is potential energy.
Eth = K micro + U micro is the energy associated with the motion of atoms/molecules within the
system (microscopic E ). It is one form of the “internal” energy.
Wext is the work done by external forces (environment). Q is the heat transferred to the
system from its environment. Work and heat are the energies transferred between systems and
the environment. They are NOT the state variables or state functions! Heat is transferred by
Chapter 15 Heat, the First Law of Thermodynamics
114
one of the following three mechanisms:
(a)
Thermal conduction
(b)
Convection
(c)
Radiation
H = kA
∆T
∆x
I = σT 4
15.1.1 Work done on/by ideal-gas processes
Vf
The work done on a gas is defined by W = − ∫ pdV . It is the negative of the area under the curve
Vi
between Vi and V f !
(a) Isochoric process ( V = const ): W = 0
(b) Isobaric process ( p = const ): W = − p∆V , ∆V = V f − Vi
(c) Isothermal process ( T = const , pV = const ):
Vf
W = −∫
Vi
Vf
Vf
Vf
nRT
dV = −nRT ln( ) = − piVi ln( ) = − p f V f ln( )
V
Vi
Vi
Vi
(d) Adibatic process ( Q = 0 , pV γ = const , γ : ratio of specific heats)
Vf
W = −∫
Vi
piVi γ
dV = − piVi γ
Vγ
Vf
∫
Vi
piVi γ 1−γ
pV V
1
dV
(Vi − V f1−γ ) = i i [( i )γ −1 − 1] =
( P V − PV
=
−
i i)
γ
γ −1
γ −1 Vf
γ −1 f f
V
Chapter 15 Heat, the First Law of Thermodynamics
115
The above expression equals to nCv ∆T .
The work done during an ideal gas process depends on the path followed through the p-V
diagram! The work done during these two ideal-gas processes is not the same.
15.1.2 Heat
Heat is the energy transfer, it is process-specific.
One needs to distinguish heat from thermal energy and temperature.
•
THERMAL ENERGY is a form of energy of the system.
•
TEMPERATURE is a measure of “hotness” of the system. It is related to the thermal
energy per molecule. It is also a state variable.
•
HEAT is the energy transferred between the system and its environment as they
interact. It is NOT a particular form of energy, nor a state variable.
15.2 The First-Law of Thermodynamics
It is about the conservation of energy of a thermodynamic system. A thermodynamic system
is one where the internal energy is the only type of energy the system may have. So, we have
( ∆E mech = 0 )
∆Eint = W + Q
If the change of internal energy is solely in the form of thermal energy, then the above
Chapter 15 Heat, the First Law of Thermodynamics
116
statement becomes ∆Eth = W + Q .
15.3
Thermal Properties of Matter
Here, we look at the consequences of ∆E th to a system, be the thermal energy change due to
work W or heat Q.
•
Temperature change: ∆Eth = Mc∆T , M is the mass, and c is specific heat. Specific
heat is the amount of energy that raises the temperature of 1 kg of a substance by 1 K.
It is material specific.
If ∆Eth = Q (i.e., W = 0 ), then Q = Mc∆T .
Molar specific heat is the amount of energy that raises the temperature of 1 mol. of a
substance by 1 K. Q = nC∆T , n is the number of moles of the substance and C is
molar specific heat. For most elemental solids, C ~ 25 J
•
mol ⋅ K
.
Phase change, as characterized by a thermal energy change without changing the
temperature. (Solid ↔ Liquid ↔ Gas)
Q = ML, where M is the mass and L is the heat of transformation.
15.3.1 Heat of transformation
Heat of transformation is the amount of heat energy that causes 1 kg of a substance to
Chapter 15 Heat, the First Law of Thermodynamics
117
undergo a phase change. The heat of transformation for a phase change between a solid and a
liquid is called Heat of Fusion ( L f ).The heat of transformation for a phase change between a
liquid and a gas is called Heat of Vaporization ( Lv ).
⎧± ML f
.
Q=⎨
⎩± MLv
15.3.2 The specific heat of gases
For a gas, one needs to distinguish between the molar specific heat at constant volume Cv
and the molar specific heat at constant pressure C p , where
Q = nCv ∆T (temperature change at constant volume “A”)
Q = nC p ∆T (temperature change at constant pressure “B”)
The thermal energy of a gas is associated with temperature, so the change of thermal energy
∆Eth will be the same for any two processes that have the same ∆T . Similarly, any two
processes that change the thermal energy of the gas by ∆Eth will cause the same
temperature change ∆T . Process A and B have the same ∆T and the same ∆E th , but they
require different amounts of heat.
For process “A”, ( ∆Eth ) A = W + Q = Q = nC v ∆T
Chapter 15 Heat, the First Law of Thermodynamics
118
For process “B”, ( ∆Eth ) B = − p∆V + nC p ∆T
So, nCv ∆T = − p∆V + nC p ∆T
Since pV = nRT , ∆( pV ) = p∆V = ∆( nRT ) = nR∆T
Thus, nCv ∆T = −nR∆T + nC p ∆T
and C p = Cv + R , ∆E th = nCv ∆T .
Remarks:
1. The change in thermal energy when temperature changes by ∆T is the same for any
processes, i.e., ∆E th = nCv ∆T .
2. The heat required to bring about the temperature change depends on the process itself. It is
different for different processes. (Heat depends on path, just like the work does!)
15.3.3 More on adiabatic process
As ∆E th = Q + W = nCv ∆T , so for an adiabatic process ( Q = 0 ), W = nCv ∆T .
As that dEth = dW
nCv dT = − pdV = −nRT
Note that
dT
R dV
dV
or
=−
V
T
CV V
Cp
R C P − Cv
=
= γ − 1 , where γ =
, the specific heat ratio (>1).
Cv
Cv
Cv
Tf
Vf
dT
dV
= −(γ − 1) ∫
So ∫
T
V
Ti
Vi
ln(
Tf
Ti
) = ln(
For ideal gas, T =
Tf
Vi γ −1
V
)
= ( i )γ −1
or
Vf
Ti
Vf
pV
, so p f V fγ = piVi γ = const. .
nR
Chapter 16
From Micro to Macro, Entropy of the 2nd
Law of Thermodynamics
16.1 The Kinetic Theory of Gases
A gas consists of a vast number of atoms/molecules ceaselessly colliding with each other and
the walls of their container. For an ideal gas,
(a) these atoms/molecules (referred to as “particles”) are in RANDOM motion and obey
Newton’s laws of motion;
(b) the total number of the particles is “large” and yet the volume occupied by these
particles is negligibly small comparing to the volume the gas occupies;
(c) no force acts on a molecule except during collision;
(d) all collisions are elastic and of negligible duration.
16.1.1
Maxwell speed Distribution
Particles in a gas move randomly with different speeds. The distribution of speeds is
described by the so-called Maxwell speed distribution:
3
m 2 2 − mv 2 2 kT
N ( v ) = 4πN (
) v e
2πkT
∞
N = ∫ N (v)dv
0
Chapter 16 From Micro to Macro, Entropy of the 2nd Law of Thermodynamics
120
1 2
mv , one may derive the Distribution molecule energies, the so-called
2
Note as E =
Maxwell-Boltzman energy distribution:
N( E ) =
2N
1
π ( kT )
1
3
2
E e
−
E
kT
2
∞
N = ∫ N ( E )dE
0
2 kT
m
•
The most probable speed ( dN dv = 0 ): v p =
•
The average speed v avg =
•
The root-mean-square (RMS) speed v rms = ( v 2 )avg =
( v 2 )avg =
1∞
∫ vN ( v )dv =
N0
1∞ 2
3 kT
v N ( v )dv =
∫
N0
m
8 kT
πm
3 kT
m
Chapter 16 From Micro to Macro, Entropy of the 2nd Law of Thermodynamics
16.1.2
Mean Free Path (MFP): the average distance between collision
1
λ=
16.1.3
121
2πd ( N v )
2
=
1
kT
2πd p
2
Microscopic origin of PRESSURE
The pressure of a gas exerted on the walls of a container in due to the steady rain of a vast
number of atoms/molecules striking the walls.
•
The force (averaged) exerted to the wall by a single atom upon collision is
Favg =
•
2 mv avg
∆t coll
The total force due to collision of all atoms/molecules
N
N
m( v 2 )avg A
m( v x2 )avg A =
3V
V
N
2
mvrms
The pressure then is p =
= NkT V
3V
Fnet = N coll Favg =
•
16.1.4
Microscopic View of TEMPERATURE
The average translational kinetic energy per molecule is
ε avg =
1
1 2
m( v 2 )avg = mv rms
2
2
∵ v rms =
3 kT
3
∴ ε avg = kT
2
m
So, temperature is simply a measure of translational kinetic energy per molecule!
16.2 Thermal energy and specific heat
Thermal energy Eth = K micro + U micro
•
Monatomic Gases, U micro = 0
Eth = K micro = N ε avg =
So, CV =
3
3
NkT = nRT
2
2
3
R = 12.5 J
mol ⋅ K
2
For a monatomic gas, the energy is exclusively translational. As the translational motions are
Chapter 16 From Micro to Macro, Entropy of the 2nd Law of Thermodynamics
122
INDEPENDENT on the three space-coordinate axes, the energy is stored in 3 independent
modes (degree of freedom).
The thermal energy of a system of particles is equally divided among all the possible energy
modes. For a system of N particles at temperature T , the energy stored in each mode (or
1
1
NkT or, in terms of moles, nRT .
2
2
1
A monatomic gas has 3 degree of freedoms, so Eth = 3 × nRT .
2
in each degree of freedom) is
Remarks: Solids: an atom in solid has 3 degrees of freedom associated with the vibrational
kinetic energy, another 3 modes associated with the stretch/compress of the bonds (potential
1
energy), so Eth = 6 × nRT = 3nRT , C = 3R = 25.0 J
.
mol ⋅ K
2
•
Diatomic molecules
(a) 3 modes for translational kinetic energy
(b) 3 modes for rotational degrees of freedom
(c) 2 modes for stretching/compressing bonds
1
So, it would suggest Eth = 8 × nRT , and C = 4 R which is inconsistent with
2
experiment. The reason is due to the “quantum effect”, which prevent 3 modes from
being active. So, for diatomic molecules
Eth =
5
5
nRT , C = R = 20.8 J
mol ⋅ K
2
2
Chapter 16 From Micro to Macro, Entropy of the 2nd Law of Thermodynamics
123
16.3 Thermal interaction & Heat
Atoms in system 1 have higher kinetic energy than those in system 2 ( T1 > T2 ), upon collision
between a “fast” atom in 1 and a slow atom in 2, energy is transferred. So, heat is the energy
transferred via collisions between the more energetic (warmer) atoms on one side and the less
energetic (cooler) atoms on the other.
At thermal equilibrium, there is no net energy transfer, or atoms at both sides have the same
average translational kinetic energy.
(ε 1 ) avg = (ε 2 ) avg
∵ ε avg =
3
kT
2
∴ T1 = T2 = T f (at thermal equilibrium)
Two thermally interacting systems reach a common final temperature by exchanging energy
via collisions, until atoms on each side have, on average, equal translational kinetic energies!
∵ Eth = Nε avg
Therefore, at equilibrium,
then E1 f =
E1 f
N1
=
E2 f
N2
=
Etot
, Etot = E1i + E 2i
N1 + N 2
N1
N2
Etot .
Etot , E2 f =
N1 + N 2
N1 + N 2
Hence, the heat flowed from “1” to “2” is Q = ∆E2 = E2 f − E2i .
Chapter 16 From Micro to Macro, Entropy of the 2nd Law of Thermodynamics
124
16.4 Irreversible Processes, Entropy and the 2nd Law of
Thermodynamics
An irreversible process is one that happens only in one direction.
e.g., heat is transferred from the warmer side to the cooler side, but not the other way.
Why? After all, heat transfer is by collision, a micro-process that is irreversible!
It lies with the “probability”. Reversible microscopic events lend to irreversible macroscopic
behavior because some macroscopic states are vastly more probable than others.
The equilibrium is actually the MOST probable state in which to be!
•
Entropy is a variable that measures the “orderedness” of a system.
It is a measure of the probability that a macroscopic state will occur.
∆S = Q (reversible, isothermal)
T
•
The 2nd Law of Thermodynamics
The entropy of an isolated system never decreases. It either increases until the system
reaches equilibrium, or if the system is in equilibrium, stays the same. An isolated
system never spontaneously generates order out of randomness.
∆S ≥ 0
Chapter 17
Heat Engines & Refrigerators
17.1 Introduction
•
Heat engine is a device that uses a cyclical process to transform heat energy
into work.
•
Refrigerator is a device that uses work to move heat from a cold object to a hot
object.
17.2 Heat to work and work to heat
•
Energy-transfer-diagram
(1) Energy reservoir is an object or part of environment so large that its temperature
and thermal energy do not change when heat is transferred between the system
Chapter 17 Heat Engines & Refrigerators
126
and the reservoir.
(2) A reservoir at a higher temperature than the system is called Hot reservoir.
(3) A reservoir at a lower temperature than the system is called Cold reservoir.
The First Law of Thermodynamics: Q = QH − QC = W + ∆Eth
•
Work to heat is easy and straight forward, which can have 100% efficiency.
•
Heat to work is difficult, the 2nd law makes the transfer efficiency <100%.
The reason lies on the fact that for a practical device that transform heat into work
must return to its initial state at the end of the process and be ready for continued use!
17.3
Heat engines and refrigerators
For any heat engine, the closed-cycle device periodically return to its initial
conditions, so ( ∆Eth ) net = 0 for a full cycle.
Therefore, W = Qnet = Q H − QC .
The engine’s THERMAL EFFICIENCY η =
Q
W
= 1− C .
QH
QH
For a refrigerator, the close-cycle device uses external work to extract heat from a
cold reservoir and exhaust heat to a hot reservoir. Again, ∆Eth = 0 . So,
QH = QC + W .
Chapter 17 Heat Engines & Refrigerators
The refrigerator Coefficient of Performance K =
127
QC
.
W
The 2nd law suggests that there is no perfect refrigerator with K = ∞ ! It also
suggests there is no perfect heat engine with η = 1 .
For the 1st statement, K = ∞ ⇒ W = 0 , then it implies the refrigerator spontaneously
draw heat from cold reservoir to hot reservoir!
For the 2nd statement, if η = 1 , then QH 1 = W , using this work, we draw heat from a
cold reservoir, so QH 2 = QC 2 + W , which equivalently drawing heat from “cold” to
“hot” spontaneously.
17.4
Ideal-gas engines and refrigerator
We use a gas as the working substance, and the close-cycle is represented by a
closed-cycle trajectory in the p − V diagram.
The net work done for such a closed-cycle is simply the area inside the closed curve.
Chapter 17 Heat Engines & Refrigerators
128
Summary of ideal gas processes
Process
Gas Law
Isochoric
pi
Isobaric
Vi
Ti
Ti
=
pf
=
Vf
Work W
Heat Q
Thermal Energy
0
nCV ∆T
∆E th = Q
− p ∆V
nC p ∆T
∆Eth = Q + W
Q = −W
∆Eth = 0
0
∆Eth = W
Tf
Tf
V
− nRT ln( f
piVi = p f V f
Isothermal
Adiabatic
Vi
piViγ = p f V fγ
( p f V f − piVi )
TiVi γ −1 = T f V fγ −1
nCV ∆T
piVi
Any
V
− pV ln( f
Vi
Ti
=
p fVf
Tf
)
)
γ −1
∆Eth = nCV ∆T
−(area under PV curve)
Properties of monatomic and diatomic gases
Monatomic
Diatomic
Eth
3
nRT
2
5
nRT
2
CV
3
R
2
5
R
2
Cp
5
R
2
7
R
2
γ
5
= 1.67
3
7
= 1 .4
5
Note: For refrigerator, as the heat always transfers from hotter object to a colder
object, it has to use the ADIBATIC process to lower the temperature of the
gas to below TC and to increase it to TH .
Chapter 17 Heat Engines & Refrigerators
17.5
129
The Carnot Cycle and the limit of efficiency
A perfectly reversible engine is one that can be operated as either a heat engine or a
refrigerator between the same two energy reservoirs and with the same energy transfer,
with only their direction changed.
A perfectly reversible engine has MAXIMUM efficiency. Otherwise, it again violate
the 2nd law.
Similarly, no refrigerator can have a coefficient of performance larger than that of a
perfectly reversible refrigerator.
A perfectly reversible engine must use only two types of processes:
(1) Frictionless mechanical interactions with no heat transfer, Q = 0
(2) Thermal interactions in which heat is transferred in an isothermal process,
∆Eth = 0
The engine that uses only there two types of processes is called CARNOT engine.
Chapter 17 Heat Engines & Refrigerators
130
Carnot engine has maximum thermal efficiency η max , and it operated as a refrigerator
has the maximum coefficient of performance K max .
•
The Carnot cycle
We now analyze the Carnot cycle and find the η max .
The Carnot cycle is an ideal gas cycle that consists of two adiabatic ( Q = 0 ) and two
isothermal ( ∆Eth = 0 ) processes.
η =1−
QC
QH
QC = Q12 = nRTC ln(
V1
)
V2
Q H = Q34 = nRTH ln(
V4
)
V3
For adiabatic process, TC V2γ −1 = TH V3γ −1 , TC V1γ −1 = TH V4γ −1 .
So,
V1 V4
T
=
and η max = 1 − C .
V2 V3
TH
For refrigerator, KCarnot =
TC
.
TH − TC