property tables and charts (si units)
advertisement
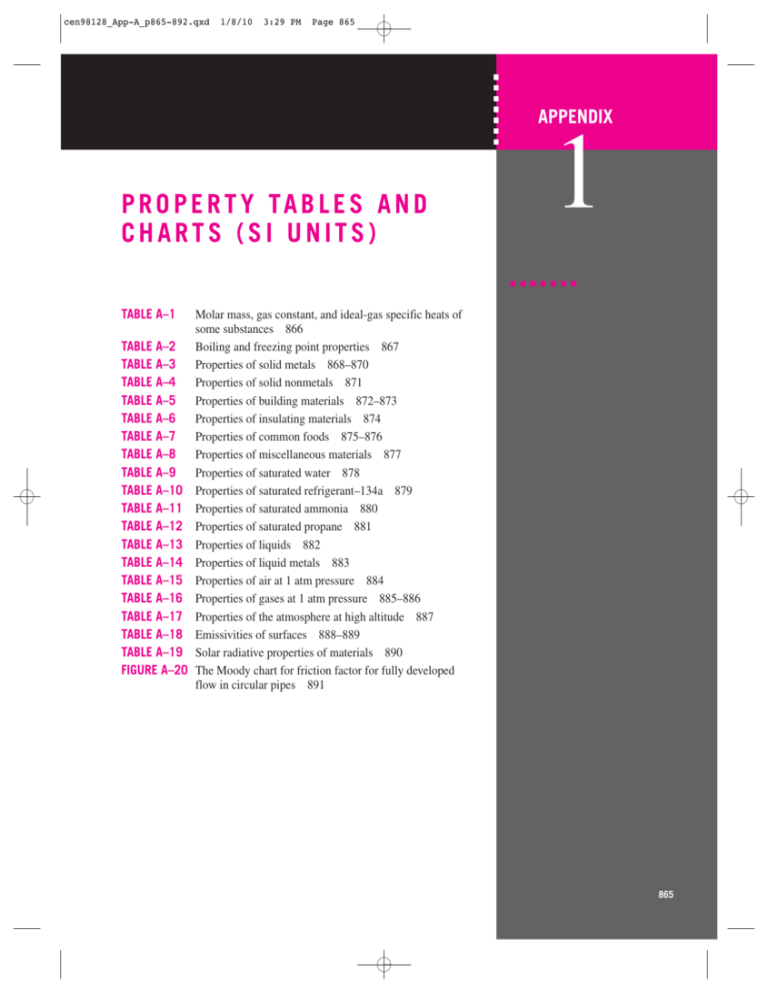
cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 865 APPENDIX P R O P E R T Y TA B L E S A N D CHARTS (SI UNITS) 1 TABLE A–1 Molar mass, gas constant, and ideal-gas specific heats of some substances 866 TABLE A–2 Boiling and freezing point properties 867 TABLE A–3 Properties of solid metals 868–870 TABLE A–4 Properties of solid nonmetals 871 TABLE A–5 Properties of building materials 872–873 TABLE A–6 Properties of insulating materials 874 TABLE A–7 Properties of common foods 875–876 TABLE A–8 Properties of miscellaneous materials 877 TABLE A–9 Properties of saturated water 878 TABLE A–10 Properties of saturated refrigerant–134a 879 TABLE A–11 Properties of saturated ammonia 880 TABLE A–12 Properties of saturated propane 881 TABLE A–13 Properties of liquids 882 TABLE A–14 Properties of liquid metals 883 TABLE A–15 Properties of air at 1 atm pressure 884 TABLE A–16 Properties of gases at 1 atm pressure 885–886 TABLE A–17 Properties of the atmosphere at high altitude 887 TABLE A–18 Emissivities of surfaces 888–889 TABLE A–19 Solar radiative properties of materials 890 FIGURE A–20 The Moody chart for friction factor for fully developed flow in circular pipes 891 865 cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 866 866 APPENDIX 1 TABLE A–1 Molar mass, gas constant, and ideal-gas specific heats of some substances Specific Heat Data at 25⬚C Substance Air Ammonia, NH3 Argon, Ar Bromine, Br2 Isobutane, C4H10 n-Butane, C4H10 Carbon dioxide, CO2 Carbon monoxide, CO Chlorine, Cl2 Chlorodifluoromethane (R-22), CHCIF2 Ethane, C2H6 Ethylene, C2H4 Fluorine, F2 Helium, He n-Heptane, C7H16 n-Hexane, C6H14 Hydrogen, H2 Krypton, Kr Methane, CH4 Neon, Ne Nitrogen, N2 Nitric oxide, NO Nitrogen dioxide, NO2 Oxygen, O2 n-Pentane, C5H12 Propane, C3H8 Propylene, C3H6 Steam, H2O Sulfur dioxide, SO2 Tetrachloromethane, CCI4 Tetrafluoroethane (R-134a), C2H2F4 Trifluoroethane (R-143a), C2H3F3 Xenon, Xe Molar Mass M, kg/kmol 28.97 17.03 39.95 159.81 58.12 58.12 44.01 28.01 70.905 86.47 30.070 28.054 38.00 4.003 100.20 86.18 2.016 83.80 16.04 20.183 28.01 30.006 46.006 32.00 72.15 44.097 42.08 18.015 64.06 153.82 102.03 84.04 131.30 Gas Constant R, kJ/kg·K* 0.2870 0.4882 0.2081 0.05202 0.1430 0.1430 0.1889 0.2968 0.1173 0.09615 0.2765 0.2964 0.2187 2.077 0.08297 0.09647 4.124 0.09921 0.5182 0.4119 0.2968 0.2771 0.1889 0.2598 0.1152 0.1885 0.1976 0.4615 0.1298 0.05405 0.08149 0.09893 0.06332 cp, kJ/kg·K cv, kJ/kg·K k ⫽ cp /cv 1.005 2.093 0.5203 0.2253 1.663 1.694 0.8439 1.039 0.4781 0.6496 1.744 1.527 0.8237 5.193 1.649 1.654 14.30 0.2480 2.226 1.030 1.040 0.9992 0.8060 0.9180 1.664 1.669 1.531 1.865 0.6228 0.5415 0.8334 0.9291 0.1583 0.7180 1.605 0.3122 0.1732 1.520 1.551 0.6550 0.7417 0.3608 0.5535 1.468 1.231 0.6050 3.116 1.566 1.558 10.18 0.1488 1.708 0.6180 0.7429 0.7221 0.6171 0.6582 1.549 1.480 1.333 1.403 0.4930 0.4875 0.7519 0.8302 0.09499 1.400 1.304 1.667 1.300 1.094 1.092 1.288 1.400 1.325 1.174 1.188 1.241 1.362 1.667 1.053 1.062 1.405 1.667 1.303 1.667 1.400 1.384 1.306 1.395 1.074 1.127 1.148 1.329 1.263 1.111 1.108 1.119 1.667 *The unit kJ/kg·K is equivalent to kPa·m3/kg·K. The gas constant is calculated from R ⫽ RU /M, where RU ⫽ 8.31447 kJ/kmol·K is the universal gas constant and M is the molar mass. Source: Specific heat values are obtained primarily from the property routines prepared by The National Institute of Standards and Technology (NIST), Gaithersburg, MD. cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 867 867 APPENDIX 1 TABLE A–2 Boiling and freezing point properties Boiling Data at l atm Freezing Data Substance Normal Latent Heat of Boiling Vaporization Point, ⬚C hfg, kJ/kg Freezing Point, ⬚C Ammonia ⫺33.3 ⫺77.7 1357 Latent Heat of Fusion Temperature, Density hif, kJ/kg ⬚C r, kg/m3 322.4 Argon Benzene Brine (20% sodium chloride by mass) n-Butane Carbon dioxide Ethanol Ethyl alcohol Ethylene glycol Glycerine Helium Hydrogen Isobutane Kerosene Mercury Methane ⫺185.9 80.2 161.6 394 ⫺189.3 5.5 103.9 ⫺0.5 ⫺78.4* 78.2 78.6 198.1 179.9 ⫺268.9 ⫺252.8 ⫺11.7 204–293 356.7 ⫺161.5 — 385.2 230.5 (at 0⬚C) 838.3 855 800.1 974 22.8 445.7 367.1 251 294.7 510.4 ⫺17.4 ⫺138.5 ⫺56.6 ⫺114.2 ⫺156 ⫺10.8 18.9 — ⫺259.2 ⫺160 ⫺24.9 ⫺38.9 ⫺182.2 109 108 181.1 200.6 — 59.5 105.7 — 11.4 58.4 Methanol Nitrogen 64.5 ⫺195.8 1100 198.6 ⫺97.7 ⫺210 99.2 25.3 124.8 306.3 ⫺57.5 180.7 ⫺218.8 13.7 ⫺187.7 80.0 Octane Oil (light) Oxygen Petroleum Propane ⫺183 — ⫺42.1 Refrigerant-134a ⫺26.1 Water 100 212.7 230–384 427.8 216.8 2257 ⫺96.6 0.0 Liquid Properties 28 126 — 80.3 — 333.7 Specific Heat cp, kJ/kg·K ⫺33.3 ⫺20 0 25 ⫺185.6 20 682 665 639 602 1394 879 4.43 4.52 4.60 4.80 1.14 1.72 20 ⫺0.5 0 25 20 20 20 ⫺268.9 ⫺252.8 ⫺11.7 20 25 ⫺161.5 ⫺100 25 ⫺195.8 ⫺160 20 25 ⫺183 20 ⫺42.1 0 50 ⫺50 ⫺26.1 0 25 0 25 50 75 100 1150 601 298 783 789 1109 1261 146.2 70.7 593.8 820 13,560 423 301 787 809 596 703 910 1141 640 581 529 449 1443 1374 1295 1207 1000 997 988 975 958 3.11 2.31 0.59 2.46 2.84 2.84 2.32 22.8 10.0 2.28 2.00 0.139 3.49 5.79 2.55 2.06 2.97 2.10 1.80 1.71 2.0 2.25 2.53 3.13 1.23 1.27 1.34 1.43 4.22 4.18 4.18 4.19 4.22 * Sublimation temperature. (At pressures below the triple-point pressure of 518 kPa, carbon dioxide exists as a solid or gas. Also, the freezing-point temperature of carbon dioxide is the triple-point temperature of ⫺56.5⬚C.) cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 868 868 APPENDIX 1 TABLE A–3 Properties of solid metals Properties at Various Temperatures (K), k(W/m·K)/cp (J/kg·K) Properties at 300 K Melting Point, K r kg/m3 cp J/kg·K k W/m·K 933 2702 903 237 97.1 Alloy 2024-T6 775 (4.5% Cu, 1.5% Mg, 0.6% Mn) Alloy 195, Cast (4.5% Cu) Beryllium 1550 2770 875 177 73.0 2790 1850 883 1825 Bismuth 545 9780 122 2573 2500 1107 27.0 594 8650 231 96.8 48.4 Chromium 2118 7160 449 93.7 29.1 Cobalt 1769 8862 421 99.2 26.6 Copper: Pure 1358 8933 385 401 117 1293 8800 420 52 14 1104 8780 355 54 17 1188 8530 380 110 1493 8920 384 23 1211 5360 322 59.9 Gold 1336 19,300 129 317 Iridium 2720 22,500 130 147 Iron: Pure 1810 7870 447 80.2 7870 447 7854 Composition Aluminum: Pure Boron Cadmium Commercial bronze (90% Cu, 10% Al) Phosphor gear bronze (89% Cu, 11% Sn) Cartridge brass (70% Cu, 30% Zn) Constantan (55% Cu, 45% Ni) Germanium Armco (99.75% pure) Carbon steels: Plain carbon (Mn ⱕ 1% Si ⱕ 0.1%) AISI 1010 168 200 7.86 a ⫻ 106 m2/s 100 68.2 59.2 6.59 9.76 200 400 600 800 302 482 65 237 798 163 240 949 186 231 1033 186 218 1146 473 787 925 1042 174 185 990 301 161 126 106 90.8 203 1114 2191 2604 2823 3018 16.5 9.69 7.04 112 120 127 190 55.5 16.8 10.6 9.60 9.85 128 600 1463 1892 2160 2338 203 99.3 94.7 198 222 242 159 111 90.9 80.7 71.3 65.4 192 384 484 542 581 616 167 122 85.4 67.4 58.2 52.1 236 379 450 503 550 628 393 397 52 160 65 — 137 395 379 417 59 545 74 — 149 425 366 433 352 451 17 237 232 190 327 109 172 90 413 356 42 785 41 — 95 360 19 362 96.8 290 323 124 153 122 43.2 337 311 131 144 133 27.3 348 298 135 138 138 19.8 357 284 140 132 144 17.4 375 270 145 126 153 23.1 134 216 94.0 384 69.5 490 54.7 574 43.3 32.8 680 975 72.7 20.7 95.6 215 80.6 384 65.7 490 53.1 574 42.2 32.3 680 975 434 60.5 17.7 7832 434 63.9 18.8 7817 446 51.9 14.9 56.7 487 58.7 559 49.8 501 48.0 39.2 30.0 559 685 1169 48.8 39.2 31.3 685 1168 44.0 37.4 29.3 582 699 971 33.9 6.71 34.7 127 50.3 482 252 75 487 Carbon–silicon (Mn ⱕ 1% 0.1% ⬍ Si ⱕ 0.6%) 1000 cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 869 869 APPENDIX 1 TABLE A–3 Properties of solid metals (Continued) Composition Melting Point, K Carbon–manganese–silicon (1% ⬍ Mn ⬍ 1.65% 0.1% ⬍ Si ⬍ 0.6%) Chromium (low) steels: 1 Cr– 41 Mo–Si (0.18% C, 2 0.65% Cr, 0.23% Mo, 0.6% Si) 1 Cr– 12 Mo (0.16% C, 1% Cr, 0.54% Mo, 0.39% Si) 1 Cr–V (0.2% C, 1.02% Cr, 0.15% V) Stainless steels: AISI 302 Properties at Various Temperatures (K), k(W/m·K)/cp(J/kg·K) Properties at 300 K r kg/m3 cp J/kg·K k W/m·K 8131 434 41.0 7822 444 7858 7836 a ⫻ 106 m2/s 100 400 600 800 11.6 42.2 487 39.7 559 35.0 27.6 685 1090 37.7 10.9 38.2 36.7 442 42.3 12.2 492 42.0 575 39.1 688 969 34.5 27.4 443 48.9 14.1 492 46.8 575 42.1 688 969 36.3 28.2 492 575 688 8055 480 15.1 3.91 7900 477 14.9 3.95 AISI 316 8238 468 13.4 3.48 AISI 347 7978 480 14.2 3.71 35.3 AISI 304 1670 Lead 601 11,340 129 Magnesium 923 1740 1024 156 87.6 Molybdenum 2894 10,240 251 138 53.7 Nickel: Pure 1728 8900 444 90.7 1672 8400 420 12 1665 8510 439 11.7 3.1 2741 8570 265 53.7 23.6 Palladium 1827 12,020 244 71.8 24.5 Platinum: Pure 2045 21,450 133 71.6 25.1 Alloy 60Pt–40Rh (60% Pt, 40% Rh) Rhenium 1800 16,630 162 47 17.4 3453 21,100 136 47.9 16.7 Rhodium 2236 12,450 243 Nichrome (80% Ni, 20% Cr) Inconel X-750 (73% Ni, 15% Cr, 6.7% Fe) Niobium 150 24.1 23.0 232 3.4 49.6 200 9.2 272 12.6 402 39.7 118 169 649 179 141 36.7 125 159 934 143 224 164 383 107 485 17.3 512 16.6 515 15.2 504 15.8 513 34.0 132 153 1074 134 261 33.3 20.0 22.8 559 585 19.8 22.6 557 582 18.3 21.3 550 576 18.9 21.9 559 585 31.4 142 149 146 1170 1267 126 118 275 285 65.6 530 16 525 17.0 67.6 562 21 545 20.5 1000 26.9 969 25.4 606 25.4 611 24.2 602 24.7 606 112 295 8.7 10.3 80.2 592 14 480 13.5 71.8 — 55.2 188 76.5 168 372 52.6 249 71.6 227 473 55.2 274 73.6 251 510 58.2 283 79.7 261 546 626 61.3 64.4 292 301 86.9 94.2 271 281 77.5 100 72.6 125 58.9 97 186 147 51.0 127 154 220 71.8 136 52 — 46.1 139 146 253 73.2 141 59 — 44.2 145 136 274 75.6 146 65 — 44.1 151 127 293 24.0 78.7 152 69 — 44.6 156 121 311 cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 870 870 APPENDIX 1 TABLE A–3 Properties of solid metals (Concluded) Properties at Various Temperatures (K), k(W/m·K)/cp (J/kg·K) Properties at 300 K Melting Point, K r kg/m3 cp J/kg·K k W/m·K Silicon 1685 2330 712 148 Silver 1235 10,500 235 429 Tantalum 3269 16,600 140 57.5 24.7 Thorium 2023 11,700 118 54.0 39.1 505 7310 227 66.6 40.1 Titanium 1953 4500 522 21.9 Tungsten 3660 19,300 132 Uranium 1406 19,070 116 27.6 12.5 Vanadium 2192 6100 489 30.7 10.3 693 7140 389 2125 6570 278 Composition Tin Zinc Zirconium 174 116 22.7 a ⫻ 106 m2/s 100 89.2 174 9.32 68.3 41.8 12.4 884 259 444 187 59.2 110 59.8 99 85.2 188 30.5 300 208 87 21.7 94 35.8 258 117 297 33.2 205 200 400 600 800 1000 264 556 430 225 57.5 133 54.6 112 73.3 215 24.5 465 186 122 25.1 108 31.3 430 118 367 25.2 264 98.9 790 425 239 57.8 144 54.5 124 62.2 243 20.4 551 159 137 29.6 125 31.3 515 111 402 21.6 300 61.9 867 412 250 58.6 146 55.8 134 42.4 913 396 262 59.4 149 56.9 145 31.2 946 379 277 60.2 152 56.9 156 19.4 591 137 142 34.0 146 33.3 540 103 436 20.7 332 19.7 633 125 146 38.8 176 35.7 563 20.7 675 118 148 43.9 180 38.2 597 21.6 23.7 342 362 From Frank P. Incropera and David P. DeWitt, Fundamentals of Heat and Mass Transfer, 3rd ed., 1990. This material is used by permission of John Wiley & Sons, Inc. cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 871 871 APPENDIX 1 TABLE A–4 Properties of solid nonmetals Composition Aluminum oxide, sapphire Aluminum oxide, polycrystalline Beryllium oxide Boron Melting Point, K r cp k a ⫻ 106 kg/m3 J/kg·K W/m·K m2/s 2323 3970 765 46 15.1 2323 3970 765 36.0 11.9 2725 3000 1030 2573 2500 1105 Boron fiber epoxy 590 (30% vol.) composite k, || to fibers k, ⬜ to fibers cp Carbon Amorphous 1500 2080 Diamond, type lla — insulator Graphite, pyrolytic 2273 k, || to layers k, ⬜ to layers cp Graphite fiber 450 epoxy (25% vol.) composite k, heat flow || to fibers k, heat flow ⬜ to fibers cp Pyroceram, 1623 Corning 9606 Silicon carbide 3100 Silicon dioxide, 1883 crystalline (quartz) k, || to c-axis k,⬜ to c-axis cp Silicon dioxide, 1883 polycrystalline (fused silica) Silicon nitride 2173 272 27.6 9.99 1122 1950 3500 — 1.60 100 200 400 600 450 — 133 — 82 — 55 — 190 — 52.5 — 32.4 940 26.4 940 196 1350 18.7 1490 18.9 1110 15.8 1110 111 1690 11.3 1880 88.0 2.29 0.59 — 509 2300 2.10 0.37 364 2.23 0.49 757 2.28 0.60 1431 0.67 — 1.18 — 1.89 — 10,000 21 4000 194 1540 853 3230 9.23 411 1390 4.09 992 21.9 — 800 1000 13.0 1180 10.4 1180 70 1865 8.1 2135 10.5 1225 7.85 1225 47 1975 6.3 2350 2.37 — 2.53 — 2210 1950 5.70 4970 16.8 136 709 892 2.68 1406 667 534 2.01 1.60 1650 1793 1216 3.64 908 — 880 3.28 1038 — 1050 3.08 2.96 1122 1197 — 87 1135 1195 7.6 4.70 885 1.51 5.0 3.4 1075 1.75 4.2 3.1 1250 2.17 905 13.9 778 1040 11.3 937 1105 1155 9.88 8.76 1063 1155 1400 2600 11.1 0.87 935 808 3.98 3160 675 490 0.46 1.89 5.7 0.68 337 5.25 — 8.7 1.1 642 4.78 — 39 20.8 — 0.69 16.4 9.5 — 1.14 230 13.0 2650 10.4 6.21 2220 745 745 2400 691 392 2070 708 Thorium dioxide 3573 9110 235 Titanium dioxide, polycrystalline 2133 4157 710 Sulfur Properties at Various Temperatures (K), k (W/m·K)/cp (J/kg·K) Properties at 300 K 1.38 0.834 — — — — — 578 0.206 0.141 0.165 0.185 403 606 13 6.1 16.0 8.4 9.65 2.8 10.2 255 7.01 805 6.6 274 5.02 880 4.7 285 8.94 910 2.87 3.68 295 3.46 930 cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 872 872 APPENDIX 1 TABLE A–5 Properties of building materials (at a mean temperature of 24⬚C) Material Building Boards Asbestos–cement board Gypsum of plaster board Plywood (Douglas fir) Insulated board and sheating (regular density) Hardboard (high density, standard tempered) Particle board: Medium density Underlayment Wood subfloor Specific Heat, cp kJ/kg·K R-value (for listed thickness, L/k), K·m2/W Thickness, L mm Density, r kg/m3 Thermal Conductivity, k W/m·K 6 mm 10 mm 13 mm — 6 mm 10 mm 13 mm 20 mm 13 mm 20 mm 1922 800 800 545 545 545 545 545 288 288 — — — 0.12 — — — — — — 1.00 1.09 — 1.21 1.21 1.21 1.21 1.21 1.30 1.30 0.011 0.057 0.078 — 0.055 0.083 0.110 0.165 0.232 0.359 — 1010 0.14 1.34 — — 16 mm 20 mm 800 640 — 0.14 — — 1.30 1.21 1.38 — 0.144 0.166 Building Membrane Vapor-permeable felt Vapor-seal (2 layers of mopped 0.73 kg/m2 felt) — — — — 0.011 — — — — 0.021 Flooring Materials Carpet and fibrous pad Carpet and rubber pad Tile (asphalt, linoleum, vinyl) — — — — — — — — — 1.42 1.38 1.26 0.367 0.217 0.009 100 mm 200 mm 300 mm 1922 2082 2400 1920 1120 — — — 0.72 1.30 1.34 0.90 0.41 0.77 1.0 1.30 — — — 0.79 — — — — — — — — — 0.13 0.20 0.23 1920 1600 1280 960 0.18 1920 1280 1857 1.1 0.79 0.54 0.33 — 1.40 0.65 0.72 — 0.84 0.84 — — — — — — — — — Masonry Materials Masonry units: Brick, common Brick, face Brick, fire clay Concrete blocks (3 oval cores, sand and gravel aggregate) Concretes: Lightweight aggregates, (including expanded shale, clay, or slate; expanded slags; cinders; pumice; and scoria) 940 Cement/lime, mortar, and stucco Stucco — — — cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 873 873 APPENDIX 1 TABLE A–5 Properties of building materials (Concluded) (at a mean temperature of 24⬚C) Specific Heat, cp kJ/kg·K R-value (for listed thickness, L/k), K·m2/W Thickness, L mm Density, r kg/m3 Thermal Conductivity, k W/m·K 10 mm 13 mm 1900 1100 1100 1100 — — — — — — 1.00 1.51 1.26 1.46 1.26 0.037 0.026 0.077 0.058 0.009 — — 1.30 0.166 19 mm 1860 0.72 0.84 0.026 13 mm 13 mm — 720 1680 720 — 0.81 0.22 — 0.84 1.34 0.055 0.016 — — 11 mm 25 mm 10 mm 1900 — — — — — — — — 1.17 1.30 1.21 0.037 0.12 0.139 0.111 10 mm 10 mm — — — 2530 — — 1.0 1.22 1.34 0.84 0.11 0.32 0.018 Woods Hardwoods (maple, oak, etc.) Softwoods (fir, pine, etc.) — — 721 513 0.159 0.115 1.26 1.38 — — Metals Aluminum (1100) Steel, mild Steel, Stainless — — — 2739 7833 7913 222 45.3 15.6 0.896 0.502 0.456 — — — Material Roofing Asbestos-cement shingles Asphalt roll roofing Asphalt shingles Built-in roofing Slate Wood shingles (plain and plastic/film faced) Plastering Materials Cement plaster, sand aggregate Gypsum plaster: Lightweight aggregate Sand aggregate Perlite aggregate Siding Material (on flat surfaces) Asbestos-cement shingles Hardboard siding Wood (drop) siding Wood (plywood) siding lapped Aluminum or steel siding (over sheeting): Hollow backed Insulating-board backed Architectural glass Source: Table A–5 and A–6 are adapted from ASHRAE, Handbook of Fundamentals (Atlanta, GA: American Society of Heating, Refrigerating, and AirConditioning Engineers, 1993), Chap. 22, Table 4. Used with permission. cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 874 874 APPENDIX 1 TABLE A–6 Properties of insulating materials (at a mean temperature of 24⬚C) Material Blanket and Batt Mineral fiber (fibrous form processed from rock, slag, or glass) Density, r kg/m3 50 to 70 mm 75 to 90 mm 135 to 165 mm 4.8–32 4.8–32 4.8–32 — — — 0.71–0.96 0.71–0.96 0.71–0.96 1.23 1.94 3.32 136 64–144 16 24 16 72 240 120 0.055 0.036 0.040 0.023 0.052 0.032 0.042 0.039 1.0 0.96 1.2 1.6 1.26 1.68 0.71 1.80 — — — — — — — — 24–40 56–72 70 0.023–0.026 0.038–0.039 0.026 — — 1.045 — — — 190 0.046 — — 9.6–32 9.6–32 — — 122 122 32–66 128–240 37–51 — — — — 0.025 0.068 0.039–0.045 0.065 0.039–0.046 0.71 0.71 0.71 0.71 — — 1.09 1.38 — 1.94 3.35 3.87 5.28 — — — — — 144 — — — 0.058 — — — 1.0 1.0 2.1 3.9 — 0.24 0.49 0.93 160 0.0017 — — 40 0.00016 — — 120 0.000017 — — Sprayed or Formed in Place Polyurethane foam Glass fiber Urethane, two-part mixture (rigid foam) Mineral wool granules with asbestos/ inorganic binders (sprayed) ~75 to 125 mm ~165 to 222 mm ~191 to 254 mm ~185 mm Silica aerogel Vermiculite (expanded) Perlite, expanded Sawdust or shavings Cellulosic insulation (milled paper or wood pulp) Roof Insulation Cellular glass Preformed, for use above deck R-value (for listed thickness, L/k), K·m2/W Thickness, L mm Board and Slab Cellular glass Glass fiber (organic bonded) Expanded polystyrene (molded beads) Expanded polyurethane (R-11 expanded) Expanded perlite (organic bonded) Expanded rubber (rigid) Mineral fiber with resin binder Cork Loose Fill Mineral fiber (rock, slag, or glass) Specific Heat, cp kJ/kg·K Thermal Conductivity, k W/m·K — 13 mm 25 mm 50 mm Reflective Insulation Silica powder (evacuated) Aluminum foil separating fluffy glass mats; 10–12 layers (evacuated); for cryogenic applications (150 K) Aluminum foil and glass paper laminate; 75–150 layers (evacuated); for cryogenic applications (150 K) 84 85 65 75 82 92 84 80 23 78 89 82 89 75 87 Fruits Apples Apricots Avocados Bananas Blueberries Cantaloupes Cherries, sour Cherries, sweet Figs, dried Figs, fresh Grapefruit Grapes Lemons Olives Oranges 3.65 3.69 3.02 3.35 3.59 3.92 3.65 3.52 — 3.45 3.82 3.59 3.82 3.35 3.75 3.65 3.96 3.82 3.86 3.92 3.79 3.92 3.99 3.32 4.06 3.96 3.35 3.69 4.02 3.89 3.86 3.82 3.79 3.69 3.32 3.92 3.45 3.89 3.96 3.99 3.92 Above Freezing 1.90 1.91 1.66 1.78 1.87 2.00 1.90 1.85 1.13 1.82 1.96 1.87 1.96 1.78 1.94 1.90 2.01 1.96 1.97 2.00 1.95 2.00 2.02 1.77 2.05 2.01 1.78 1.91 2.04 1.99 1.97 1.96 1.95 1.91 1.77 2.00 1.82 1.99 2.01 2.02 2.00 Below Freezing 281 284 217 251 274 307 281 267 77 261 297 274 297 251 291 281 311 297 301 307 294 307 314 247 321 311 251 284 317 304 301 297 294 284 247 307 261 304 311 314 307 49 70 67 56 74 65 37 56 38 64 Meats Beef carcass Liver Round, beef Sirloin, beef Chicken Lamb leg Port carcass Ham Pork sausage Turkey 5 16 37 39 1 74 17 63 88 6 2 3 4 78 75 79 57 64 83 Fish/Seafood Cod, whole Halibut, whole Lobster Mackerel Salmon, whole Shrimp Other Almonds Butter Cheese, Cheddar Cheese, Swiss Chocolate milk Eggs, whole Honey Ice cream Milk, whole Peanuts Peanuts, roasted Pecans Walnuts 89 83 85 86 85 18 90 87 93 Peaches Pears Pineapples Plums Quinces Raisins Strawberries Tangerines Watermelon Water content,a %(mass) — — ⫺12.9 ⫺10.0 — ⫺0.6 — ⫺5.6 ⫺0.6 — — — — ⫺1.7 ⫺1.7 — — ⫺2.8 — — ⫺1.7 — — — — 2.08 2.15 — 3.32 — 2.95 3.79 — — — — 2.48 3.18 3.08 2.72 3.32 3.02 2.08 2.72 2.11 2.98 3.45 3.35 3.49 2.75 2.98 3.62 3.82 3.62 3.69 3.72 3.69 — 3.86 3.75 3.96 ⫺0.9 ⫺1.6 ⫺1.0 ⫺0.8 ⫺2.0 — ⫺0.8 ⫺1.1 ⫺0.4 ⫺2.2 ⫺2.2 ⫺2.2 ⫺2.2 ⫺2.2 ⫺2.2 Above Freezing Freezing Pointa ⬚C 0.89 1.04 1.31 1.33 0.85 1.77 1.05 1.63 1.95 0.92 0.87 0.87 0.88 1.46 1.72 1.68 1.55 1.77 1.66 1.31 1.55 1.32 1.65 1.82 1.78 1.84 1.56 1.65 1.89 1.96 1.89 1.91 1.92 1.91 1.07 1.97 1.94 2.01 Below Freezing Specific heat,b kJ/kg·K 17 53 124 130 3 247 57 210 294 20 7 10 13 164 234 224 187 247 217 124 187 127 214 261 251 264 190 214 277 297 277 284 287 284 60 301 291 311 Latent Heat of Fusion,c kJ/kg Specific heat data are based on the specific heat values of a water and ice at 0⬚C and are determined from Siebel’s formulas: cp, fresh ⫽ 3.35 ⫻ (Water content) ⫹ 0.84, above freezing, and cp, frozen ⫽ 1.26 ⫻ (Water content) ⫹0.84, below freezing. The latent heat of fusion is determined by multiplying the heat of fusion of water (334 kJ/kg) by the water content of the food. c b 3:29 PM ⫺1.1 ⫺1.1 ⫺0.3 ⫺0.8 ⫺1.6 ⫺1.2 ⫺1.7 ⫺1.8 — ⫺2.4 ⫺1.1 ⫺1.1 ⫺1.4 ⫺1.4 ⫺0.8 ⫺1.2 ⫺0.6 ⫺0.7 ⫺0.6 ⫺0.9 ⫺1.4 ⫺0.8 ⫺0.5 ⫺0.6 ⫺0.5 ⫺0.8 ⫺1.8 ⫺0.7 ⫺0.2 ⫺0.9 ⫺1.8 ⫺0.9 ⫺0.8 ⫺1.1 ⫺0.6 ⫺0.7 ⫺0.6 ⫺0.8 ⫺0.3 ⫺0.5 ⫺1.1 Freezing Pointa ⬚C Latent Heat of Fusion,c kJ/kg Food 1/8/10 Sources: aWater content and freezing-point data are from ASHRAE, Handbook of Fundamentals, Sl version (Atlanta, GA: American Society of Heating, Refrigerating and Air–Conditioning Engineers, Inc., 1993), Chap. 30. Table 1. Used with permission. Freezing point is the temperature at which freezing starts for fruits and vegetables, and the average freezing temperature for other foods. 84 93 89 90 92 88 92 94 74 96 93 75 85 95 91 90 89 88 85 74 92 78 91 93 94 92 Vegetables Artichokes Asparagus Beans, snap Broccoli Cabbage Carrots Cauliflower Celery Corn, sweet Cucumbers Eggplant Horseradish Leeks Lettuce Mushrooms Okra Onions, green Onions, dry Parsley Peas, green Peppers, sweet Potatoes Pumpkins Spinach Tomatos, rlpe Turnips Food Water content,a %(mass) Specific heat,b kJ/kg·K Properties of common foods (a) Specific heats and freezing-point properties TABLE A–7 cen98128_App-A_p865-892.qxd Page 875 875 APPENDIX 1 cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 876 876 APPENDIX 1 TABLE A–7 Properties of common foods (Concluded) (b) Other properties Water Content, % (mass) Temperature, T ⬚C Density, r kg/m3 Thermal Conductivity, k W/m·K Thermal Diffusivity, a m2/s Specific Heat, cp kJ/kg·K Fruits/Vegetables Apple juice Apples Apples, dried Apricots, dried Bananas, fresh Broccoli Cherries, fresh Figs Grape juice Peaches Plums Potatoes Raisins 87 85 41.6 43.6 76 — 92 40.4 89 89 — 78 32 20 8 23 23 27 ⫺6 0–30 23 20 2–32 ⫺16 0–70 23 1000 840 856 1320 980 560 1050 1241 1000 960 610 1055 1380 0.559 0.418 0.219 0.375 0.481 0.385 0.545 0.310 0.567 0.526 0.247 0.498 0.376 0.14 ⫻ 10⫺6 0.13 ⫻ 10⫺6 0.096 ⫻ 10⫺6 0.11 ⫻ 10⫺6 0.14 ⫻ 10⫺6 — 0.13 ⫻ 10⫺6 0.096 ⫻ 10⫺6 0.14 ⫻ 10⫺6 0.14 ⫻ 10⫺6 — 0.13 ⫻ 10⫺6 0.11 ⫻ 10⫺6 3.86 3.81 2.72 2.77 3.59 — 3.99 2.69 3.91 3.91 — 3.64 2.48 Meats Beef, ground Beef, lean Beef fat Beef liver Cat food Chicken breast Dog food Fish, cod Fish, salmon Ham Lamb Pork, lean Turkey breast Veal 67 74 0 72 39.7 75 30.6 81 67 71.8 72 72 74 75 6 3 35 35 23 0 23 3 3 20 20 4 3 20 950 1090 810 — 1140 1050 1240 1180 — 1030 1030 1030 1050 1060 0.406 0.471 0.190 0.448 0.326 0.476 0.319 0.534 0.531 0.480 0.456 0.456 0.496 0.470 0.13 0.13 — — 0.11 0.13 0.11 0.12 — 0.14 0.13 0.13 0.13 0.13 16 31.9 16 91 88 0 0 100 100 32.3 4 23 5 20 28 32 4 0 30 23 — 340 1000 — — 910 920 1000 995 450 0.197 0.106 0.233 0.566 0.580 0.168 0.168 0.569 0.618 0.082 — 0.12 0.11 — — — — 0.14 0.15 0.10 Food Other Butter Chocolate cake Margarine Milk, skimmed Milk, whole Olive oil Peanut oil Water White cake ⫻ 10⫺6 ⫻ 10⫺6 ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺6 10⫺6 10⫺6 ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 ⫻ 10⫺6 ⫻ 10⫺6 ⫻ 10⫺6 ⫻ 10⫺6 ⫻ 10⫺6 3.36 3.54 — 3.49 2.68 3.56 2.45 3.71 3.36 3.48 3.49 3.49 3.54 3.56 2.08 2.48 2.08 3.96 3.89 — — 4.217 4.178 2.49 Source: Data obtained primarily from ASHRAE, Handbook of Fundamentals, SI version (Atlanta, GA: American Society of Heating, Refrigerating and AirConditioning Engineers, Inc., 1993), Chap. 30, Tables 7 and 9. Used with permission. Most specific heats are calculated from cp ⫽ 1.68 ⫹ 2.51 ⫻ (Water content), which is a good approximation in the temperature range of 3 to 32⬚C. Most thermal diffusivities are calculated from a ⫽ k/rcp. Property values given here are valid for the specific water content. cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 877 877 APPENDIX 1 TABLE A–8 Properties of miscellaneous materials (Values are at 300 K unless indicated otherwise) Material Asphalt Bakelite Brick, refractory Chrome brick 473 K 823 K 1173 K Fire clay, burnt 1600 K 773 K 1073 K 1373 K Fire clay, burnt 1725 K 773 K 1073 K 1373 K Fire clay brick 478 K 922 K 1478 K Magnesite 478 K 922 K 1478 K Chicken meat, white (74.4% water content) 198 K 233 K 253 K 273 K 293 K Clay, dry Clay, wet Coal, anthracite Concrete (stone mix) Cork Cotton Fat Glass Window Pyrex Crown Lead Thermal Density, r Conductivity, k kg/m3 W/m·K 2115 1300 0.062 1.4 3010 — — 2.3 2.5 2.0 2050 — — 1.0 1.1 1.1 Specific Heat, cp J/kg·K 920 1465 835 — — 960 — — 2325 — — 1.3 1.4 1.4 960 — — 2645 — — 1.0 1.5 1.8 960 — — — — — 3.8 2.8 1.9 1130 — — — — — — — 1550 1495 1350 1.60 1.49 1.35 0.48 0.49 0.930 1.675 0.26 — — — — — — — 1260 2300 86 80 — 1.4 0.048 0.06 0.17 880 2030 1300 — 2800 2225 2500 3400 0.7 1–1.4 1.05 0.85 750 835 — — Source: Compiled from various sources. Material Ice 273 K 253 K 173 K Leather, sole Linoleum Mica Paper Plastics Plexiglass Teflon 300 K 400 K Lexan Nylon Polypropylene Polyester PVC, vinyl Porcelain Rubber, natural Rubber, vulcanized Soft Hard Sand Snow, fresh Snow, 273 K Soil, dry Soil, wet Sugar Tissue, human Skin Fat layer Muscle Vaseline Wood, cross-grain Balsa Fir Oak White pine Yellow pine Wood, radial Oak Fir Wool, ship Thermal Density, r Conductivity, k kg/m3 W/m·K Specific Heat, cp J/kg·K 920 922 928 998 535 1180 2900 930 1.88 2.03 3.49 0.159 0.081 0.186 0.523 0.180 2040 1945 1460 — — — — 1340 1190 0.19 1465 2200 — 1200 1145 910 1395 1470 2300 1150 0.35 0.45 0.19 0.29 0.12 0.15 0.1 1.5 0.28 1050 — 1260 — 1925 1170 840 — — 1100 1190 1515 100 500 1500 1900 1600 0.13 0.16 0.2–1.0 0.60 2.2 1.0 2.0 0.58 2010 — 800 — — 1900 2200 — 0.37 0.2 0.41 0.17 — — — — — — — — 140 415 545 435 640 0.055 0.11 0.17 0.11 0.15 — 2720 2385 — 2805 545 420 145 0.19 0.14 0.05 2385 2720 — cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 878 878 APPENDIX 1 TABLE A–9 Properties of saturated water Temp. T, ⬚C 0.01 5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100 110 120 130 140 150 160 170 180 190 200 220 240 260 280 300 320 340 360 374.14 Saturation Pressure Psat, kPa 0.6113 0.8721 1.2276 1.7051 2.339 3.169 4.246 5.628 7.384 9.593 12.35 15.76 19.94 25.03 31.19 38.58 47.39 57.83 70.14 84.55 101.33 143.27 198.53 270.1 361.3 475.8 617.8 791.7 1,002.1 1,254.4 1,553.8 2,318 3,344 4,688 6,412 8,581 11,274 14,586 18,651 22,090 Density r, kg/m3 Liquid Vapor 999.8 0.0048 999.9 0.0068 999.7 0.0094 999.1 0.0128 998.0 0.0173 997.0 0.0231 996.0 0.0304 994.0 0.0397 992.1 0.0512 990.1 0.0655 988.1 0.0831 985.2 0.1045 983.3 0.1304 980.4 0.1614 977.5 0.1983 974.7 0.2421 971.8 0.2935 968.1 0.3536 965.3 0.4235 961.5 0.5045 957.9 0.5978 950.6 0.8263 943.4 1.121 934.6 1.496 921.7 1.965 916.6 2.546 907.4 3.256 897.7 4.119 887.3 5.153 876.4 6.388 864.3 7.852 840.3 11.60 813.7 16.73 783.7 23.69 750.8 33.15 713.8 46.15 667.1 64.57 610.5 92.62 528.3 144.0 317.0 317.0 Enthalpy of Vaporization Specific Heat cp, J/kg·K hfg, kJ/kg Liquid Vapor 2501 2490 2478 2466 2454 2442 2431 2419 2407 2395 2383 2371 2359 2346 2334 2321 2309 2296 2283 2270 2257 2230 2203 2174 2145 2114 2083 2050 2015 1979 1941 1859 1767 1663 1544 1405 1239 1028 720 0 4217 4205 4194 4185 4182 4180 4178 4178 4179 4180 4181 4183 4185 4187 4190 4193 4197 4201 4206 4212 4217 4229 4244 4263 4286 4311 4340 4370 4410 4460 4500 4610 4760 4970 5280 5750 6540 8240 14,690 — 1854 1857 1862 1863 1867 1870 1875 1880 1885 1892 1900 1908 1916 1926 1936 1948 1962 1977 1993 2010 2029 2071 2120 2177 2244 2314 2420 2490 2590 2710 2840 3110 3520 4070 4835 5980 7900 11,870 25,800 — Thermal Conductivity k, W/m·K Liquid Vapor 0.561 0.0171 0.571 0.0173 0.580 0.0176 0.589 0.0179 0.598 0.0182 0.607 0.0186 0.615 0.0189 0.623 0.0192 0.631 0.0196 0.637 0.0200 0.644 0.0204 0.649 0.0208 0.654 0.0212 0.659 0.0216 0.663 0.0221 0.667 0.0225 0.670 0.0230 0.673 0.0235 0.675 0.0240 0.677 0.0246 0.679 0.0251 0.682 0.0262 0.683 0.0275 0.684 0.0288 0.683 0.0301 0.682 0.0316 0.680 0.0331 0.677 0.0347 0.673 0.0364 0.669 0.0382 0.663 0.0401 0.650 0.0442 0.632 0.0487 0.609 0.0540 0.581 0.0605 0.548 0.0695 0.509 0.0836 0.469 0.110 0.427 0.178 — — Prandtl Number Pr Dynamic Viscosity m, kg/m·s Liquid 1.792 1.519 1.307 1.138 1.002 0.891 0.798 0.720 0.653 0.596 0.547 0.504 0.467 0.433 0.404 0.378 0.355 0.333 0.315 0.297 0.282 0.255 0.232 0.213 0.197 0.183 0.170 0.160 0.150 0.142 0.134 0.122 0.111 0.102 0.094 0.086 0.078 0.070 0.060 0.043 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 Volume Expansion Coefficient b, 1/K Vapor Liquid Vapor ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 13.5 11.2 9.45 8.09 7.01 6.14 5.42 4.83 4.32 3.91 3.55 3.25 2.99 2.75 2.55 2.38 2.22 2.08 1.96 1.85 1.75 1.58 1.44 1.33 1.24 1.16 1.09 1.03 0.983 0.947 0.910 0.865 0.836 0.832 0.854 0.902 1.00 1.23 2.06 1.00 ⫺0.068 ⫻ 10⫺3 1.00 0.015 ⫻ 10⫺3 1.00 0.733 ⫻ 10⫺3 1.00 0.138 ⫻ 10⫺3 1.00 0.195 ⫻ 10⫺3 1.00 0.247 ⫻ 10⫺3 1.00 0.294 ⫻ 10⫺3 1.00 0.337 ⫻ 10⫺3 1.00 0.377 ⫻ 10⫺3 1.00 0.415 ⫻ 10⫺3 1.00 0.451 ⫻ 10⫺3 1.00 0.484 ⫻ 10⫺3 1.00 0.517 ⫻ 10⫺3 1.00 0.548 ⫻ 10⫺3 1.00 0.578 ⫻ 10⫺3 1.00 0.607 ⫻ 10⫺3 1.00 0.653 ⫻ 10⫺3 1.00 0.670 ⫻ 10⫺3 1.00 0.702 ⫻ 10⫺3 1.00 0.716 ⫻ 10⫺3 1.00 0.750 ⫻ 10⫺3 1.00 0.798 ⫻ 10⫺3 1.00 0.858 ⫻ 10⫺3 1.01 0.913 ⫻ 10⫺3 1.02 0.970 ⫻ 10⫺3 1.02 1.025 ⫻ 10⫺3 1.05 1.145 ⫻ 10⫺3 1.05 1.178 ⫻ 10⫺3 1.07 1.210 ⫻ 10⫺3 1.09 1.280 ⫻ 10⫺3 1.11 1.350 ⫻ 10⫺3 1.15 1.520 ⫻ 10⫺3 1.24 1.720 ⫻ 10⫺3 1.35 2.000 ⫻ 10⫺3 1.49 2.380 ⫻ 10⫺3 1.69 2.950 ⫻ 10⫺3 1.97 2.43 3.73 0.922 0.934 0.946 0.959 0.973 0.987 1.001 1.016 1.031 1.046 1.062 1.077 1.093 1.110 1.126 1.142 1.159 1.176 1.193 1.210 1.227 1.261 1.296 1.330 1.365 1.399 1.434 1.468 1.502 1.537 1.571 1.641 1.712 1.788 1.870 1.965 2.084 2.255 2.571 4.313 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 Liquid Note 1: Kinematic viscosity n and thermal diffusivity a can be calculated from their definitions, n ⫽ m/r and a ⫽ k/rcp ⫽ n/Pr. The temperatures 0.01⬚C, 100⬚C, and 374.14⬚C are the triple-, boiling-, and critical-point temperatures of water, respectively. The properties listed above (except the vapor density) can be used at any pressure with negligible error except at temperatures near the critical-point value. Note 2: The unit kJ/kg·⬚C for specific heat is equivalent to kJ/kg·K, and the unit W/m·⬚C for thermal conductivity is equivalent to W/m·K. Source: Viscosity and thermal conductivity data are from J. V. Sengers and J. T. R. Watson, Journal of Physical and Chemical Reference Data 15 (1986), pp. 1291–1322. Other data are obtained from various sources or calculated. cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 879 879 APPENDIX 1 TABLE A–10 Properties of saturated refrigerant–134a Specific Enthalpy Density Heat of Saturation r, kg/m3 Vaporization cp, J/kg·K Temp. Pressure T, ⬚C P, kPa Liquid Vapor hfg, kJ/kg Liquid Vapor ⫺40 ⫺35 ⫺30 ⫺25 ⫺20 ⫺15 ⫺10 ⫺5 0 5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100 51.2 66.2 84.4 106.5 132.8 164.0 200.7 243.5 293.0 349.9 414.9 488.7 572.1 665.8 770.6 887.5 1017.1 1160.5 1318.6 1492.3 1682.8 1891.0 2118.2 2365.8 2635.2 2928.2 3246.9 3594.1 3975.1 1418 1403 1389 1374 1359 1343 1327 1311 1295 1278 1261 1244 1226 1207 1188 1168 1147 1125 1102 1078 1053 1026 996.2 964 928.2 887.1 837.7 772.5 651.7 2.773 3.524 4.429 5.509 6.787 8.288 10.04 12.07 14.42 17.12 20.22 23.75 27.77 32.34 37.53 43.41 50.08 57.66 66.27 76.11 87.38 100.4 115.6 133.6 155.3 182.3 217.8 269.3 376.3 225.9 222.7 219.5 216.3 213.0 209.5 206.0 202.4 198.7 194.8 190.8 186.6 182.3 177.8 173.1 168.2 163.0 157.6 151.8 145.7 139.1 132.1 124.4 115.9 106.4 95.4 82.2 64.9 33.9 1254 1264 1273 1283 1294 1306 1318 1330 1344 1358 1374 1390 1408 1427 1448 1471 1498 1529 1566 1608 1659 1722 1801 1907 2056 2287 2701 3675 7959 748.6 764.1 780.2 797.2 814.9 833.5 853.1 873.8 895.6 918.7 943.2 969.4 997.6 1028 1061 1098 1138 1184 1237 1298 1372 1462 1577 1731 1948 2281 2865 4144 8785 Thermal Conductivity k, W/m·K Liquid 0.1101 0.1084 0.1066 0.1047 0.1028 0.1009 0.0989 0.0968 0.0947 0.0925 0.0903 0.0880 0.0856 0.0833 0.0808 0.0783 0.0757 0.0731 0.0704 0.0676 0.0647 0.0618 0.0587 0.0555 0.0521 0.0484 0.0444 0.0396 0.0322 Vapor 0.00811 0.00862 0.00913 0.00963 0.01013 0.01063 0.01112 0.01161 0.01210 0.01259 0.01308 0.01357 0.01406 0.01456 0.01507 0.01558 0.01610 0.01664 0.01720 0.01777 0.01838 0.01902 0.01972 0.02048 0.02133 0.02233 0.02357 0.02544 0.02989 Liquid Vapor Volume Expansion Coefficient Surface Tension, b, l/K Liquid N/m 10 5.558 10⫺6 5.257 10⫺6 4.992 10⫺6 4.757 10⫺6 4.548 10⫺6 4.363 10⫺6 4.198 10⫺6 4.051 10⫺6 3.919 10⫺6 3.802 10⫺6 3.697 10⫺6 3.604 10⫺5 3.521 10⫺5 3.448 10⫺5 3.383 10⫺5 3.328 10⫺5 3.285 10⫺5 3.253 10⫺5 3.231 10⫺5 3.223 10⫺5 3.229 10⫺5 3.255 10⫺5 3.307 10⫺5 3.400 10⫺5 3.558 10⫺5 3.837 10⫺5 4.385 10⫺5 5.746 10⫺5 11.77 0.235 0.266 0.299 0.335 0.374 0.415 0.459 0.505 0.553 0.603 0.655 0.708 0.763 0.819 0.877 0.935 0.995 1.058 1.123 1.193 1.272 1.362 1.471 1.612 1.810 2.116 2.658 3.862 8.326 0.00205 0.00209 0.00215 0.00220 0.00227 0.00233 0.00241 0.00249 0.00258 0.00269 0.00280 0.00293 0.00307 0.00324 0.00342 0.00364 0.00390 0.00420 0.00455 0.00500 0.00554 0.00624 0.00716 0.00843 0.01031 0.01336 0.01911 0.03343 0.10047 Prandtl Number Pr Dynamic Viscosity m, kg/m·s Liquid 4.878 4.509 4.178 3.882 3.614 3.371 3.150 2.947 2.761 2.589 2.430 2.281 2.142 2.012 1.888 1.772 1.660 1.554 1.453 1.355 1.260 1.167 1.077 9.891 9.011 8.124 7.203 6.190 4.765 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ Vapor ⫺4 10 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 2.550 3.003 3.504 4.054 4.651 5.295 5.982 6.709 7.471 8.264 9.081 9.915 1.075 1.160 1.244 1.327 1.408 1.486 1.562 1.634 1.704 1.771 1.839 1.908 1.982 2.071 2.187 2.370 2.833 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫺6 0.01760 0.01682 0.01604 0.01527 0.01451 0.01376 0.01302 0.01229 0.01156 0.01084 0.01014 0.00944 0.00876 0.00808 0.00742 0.00677 0.00613 0.00550 0.00489 0.00429 0.00372 0.00315 0.00261 0.00209 0.00160 0.00114 0.00071 0.00033 0.00004 Note 1: Kinematic viscosity n and thermal diffusivity a can be calculated from their definitions, n ⫽ m/r and a ⫽ k/rcp ⫽ n/Pr. The properties listed here (except the vapor density) can be used at any pressures with negligible error except at temperatures near the critical-point value. Note 2: The unit kJ/kg·⬚C for specific heat is equivalent to kJ/kg·K, and the unit W/m·⬚C for thermal conductivity is equivalent to W/m·K. Source: Data generated from the EES software developed by S. A. Klein and F. L. Alvarado. Original sources: R. Tillner-Roth and H. D. Baehr, “An International Standard Formulation for the Thermodynamic Properties of 1,1,1,2-Tetrafluoroethane (HFC-134a) for Temperatures from 170 K to 455 K and Pressures up to 70 MPa,” J. Phys. Chem, Ref. Data, Vol. 23, No. 5, 1994; M.J. Assael, N. K. Dalaouti, A. A. Griva, and J. H. Dymond, “Viscosity and Thermal Conductivity of Halogenated Methane and Ethane Refrigerants,” IJR, Vol. 22, pp. 525–535, 1999; NIST REFPROP 6 program (M. O. McLinden, S. A. Klein, E. W. Lemmon, and A. P. Peskin, Physical and Chemical Properties Division, National Institute of Standards and Technology, Boulder, CO 80303, 1995). cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 880 880 APPENDIX 1 TABLE A–11 Properties of saturated ammonia Specific Thermal Enthalpy Density Heat Conductivity of Saturation k, W/m·K r, kg/m3 Vaporization cp, J/kg·K Temp. Pressure T, ⬚C P, kPa Liquid Vapor hfg, kJ/kg Liquid Vapor Liquid Vapor ⫺40 ⫺30 ⫺25 ⫺20 ⫺15 ⫺10 ⫺5 0 5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100 71.66 119.4 151.5 190.1 236.2 290.8 354.9 429.6 516 615.3 728.8 857.8 1003 1167 1351 1555 1782 2033 2310 2614 2948 3312 3709 4141 4609 5116 5665 6257 690.2 677.8 671.5 665.1 658.6 652.1 645.4 638.6 631.7 624.6 617.5 610.2 602.8 595.2 587.4 579.4 571.3 562.9 554.2 545.2 536.0 526.3 516.2 505.7 494.5 482.8 470.2 456.6 0.6435 1.037 1.296 1.603 1.966 2.391 2.886 3.458 4.116 4.870 5.729 6.705 7.809 9.055 10.46 12.03 13.8 15.78 18.00 20.48 23.26 26.39 29.90 33.87 38.36 43.48 49.35 56.15 1389 1360 1345 1329 1313 1297 1280 1262 1244 1226 1206 1186 1166 1144 1122 1099 1075 1051 1025 997.4 968.9 939.0 907.5 874.1 838.6 800.6 759.8 715.5 4414 4465 4489 4514 4538 4564 4589 4617 4645 4676 4709 4745 4784 4828 4877 4932 4993 5063 5143 5234 5340 5463 5608 5780 5988 6242 6561 6972 2242 2322 2369 2420 2476 2536 2601 2672 2749 2831 2920 3016 3120 3232 3354 3486 3631 3790 3967 4163 4384 4634 4923 5260 5659 6142 6740 7503 — — 0.5968 0.5853 0.5737 0.5621 0.5505 0.5390 0.5274 0.5158 0.5042 0.4927 0.4811 0.4695 0.4579 0.4464 0.4348 0.4232 0.4116 0.4001 0.3885 0.3769 0.3653 0.3538 0.3422 0.3306 0.3190 0.3075 0.01792 0.01898 0.01957 0.02015 0.02075 0.02138 0.02203 0.02270 0.02341 0.02415 0.02492 0.02573 0.02658 0.02748 0.02843 0.02943 0.03049 0.03162 0.03283 0.03412 0.03550 0.03700 0.03862 0.04038 0.04232 0.04447 0.04687 0.04958 Dynamic Viscosity m, kg/m·s Liquid 2.926 2.630 2.492 2.361 2.236 2.117 2.003 1.896 1.794 1.697 1.606 1.519 1.438 1.361 1.288 1.219 1.155 1.094 1.037 9.846 9.347 8.879 8.440 8.030 7.646 7.284 6.946 6.628 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ Vapor 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 7.957 8.311 8.490 8.669 8.851 9.034 9.218 9.405 9.593 9.784 9.978 1.017 1.037 1.057 1.078 1.099 1.121 1.143 1.166 1.189 1.213 1.238 1.264 1.292 1.322 1.354 1.389 1.429 Liquid Vapor Volume Expansion Coefficient b, l/K Liquid — — 1.875 1.821 1.769 1.718 1.670 1.624 1.580 1.539 1.500 1.463 1.430 1.399 1.372 1.347 1.327 1.310 1.297 1.288 1.285 1.287 1.296 1.312 1.338 1.375 1.429 1.503 0.9955 1.017 1.028 1.041 1.056 1.072 1.089 1.107 1.126 1.147 1.169 1.193 1.218 1.244 1.272 1.303 1.335 1.371 1.409 1.452 1.499 1.551 1.612 1.683 1.768 1.871 1.999 2.163 0.00176 0.00185 0.00190 0.00194 0.00199 0.00205 0.00210 0.00216 0.00223 0.00230 0.00237 0.00245 0.00254 0.00264 0.00275 0.00287 0.00301 0.00316 0.00334 0.00354 0.00377 0.00404 0.00436 0.00474 0.00521 0.00579 0.00652 0.00749 Prandtl Number Pr ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 Surface Tension, N/m 0.03565 0.03341 0.03229 0.03118 0.03007 0.02896 0.02786 0.02676 0.02566 0.02457 0.02348 0.02240 0.02132 0.02024 0.01917 0.01810 0.01704 0.01598 0.01493 0.01389 0.01285 0.01181 0.01079 0.00977 0.00876 0.00776 0.00677 0.00579 Note 1: Kinematic viscosity n and thermal diffusivity a can be calculated from their definitions, n ⫽ m/r and a ⫽ k/rcp ⫽ n/Pr. The properties listed here (except the vapor density) can be used at any pressures with negligible error except at temperatures near the critical-point value. Note 2: The unit kJ/kg·⬚C for specific heat is equivalent to kJ/kg·K, and the unit W/m·⬚C for thermal conductivity is equivalent to W/m·K. Source: Data generated from the EES software developed by S. A. Klein and F. L. Alvarado. Original sources: Tillner-Roth, Harms-Watzenberg, and Baehr, “Eine neue Fundamentalgleichung fur Ammoniak,” DKV-Tagungsbericht 20:167–181, 1993; Liley and Desai, “Thermophysical Properties of Refrigerants,” ASHRAE, 1993, ISBN 1-1883413-10-9. cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 881 881 APPENDIX 1 TABLE A–12 Properties of saturated propane Density Saturation r, kg/m3 Temp. Pressure T, ⬚C P, kPa Liquid Vapor ⫺120 0.4053 ⫺110 1.157 ⫺100 2.881 ⫺90 6.406 ⫺80 12.97 ⫺70 24.26 ⫺60 42.46 ⫺50 70.24 ⫺40 110.7 ⫺30 167.3 ⫺20 243.8 ⫺10 344.4 0 473.3 5 549.8 10 635.1 15 729.8 20 834.4 25 949.7 30 1076 35 1215 40 1366 45 1530 50 1708 60 2110 70 2580 80 3127 90 3769 664.7 0.01408 654.5 0.03776 644.2 0.08872 633.8 0.1870 623.2 0.3602 612.5 0.6439 601.5 1.081 590.3 1.724 578.8 2.629 567.0 3.864 554.7 5.503 542.0 7.635 528.7 10.36 521.8 11.99 514.7 13.81 507.5 15.85 500.0 18.13 492.2 20.68 484.2 23.53 475.8 26.72 467.1 30.29 458.0 34.29 448.5 38.79 427.5 49.66 403.2 64.02 373.0 84.28 329.1 118.6 Specific Enthalpy Heat of Vaporization cp, J/kg·K hfg, kJ/kg Liquid Vapor 498.3 489.3 480.4 471.5 462.4 453.1 443.5 433.6 423.1 412.1 400.3 387.8 374.2 367.0 359.5 351.7 343.4 334.8 325.8 316.2 306.1 295.3 283.9 258.4 228.0 189.7 133.2 2003 2021 2044 2070 2100 2134 2173 2217 2258 2310 2368 2433 2507 2547 2590 2637 2688 2742 2802 2869 2943 3026 3122 3283 3595 4501 6977 1115 1148 1183 1221 1263 1308 1358 1412 1471 1535 1605 1682 1768 1814 1864 1917 1974 2036 2104 2179 2264 2361 2473 2769 3241 4173 7239 Thermal Conductivity k, W/m·K Liquid 0.1802 0.1738 0.1672 0.1606 0.1539 0.1472 0.1407 0.1343 0.1281 0.1221 0.1163 0.1107 0.1054 0.1028 0.1002 0.0977 0.0952 0.0928 0.0904 0.0881 0.0857 0.0834 0.0811 0.0765 0.0717 0.0663 0.0595 Vapor 0.00589 0.00645 0.00705 0.00769 0.00836 0.00908 0.00985 0.01067 0.01155 0.01250 0.01351 0.01459 0.01576 0.01637 0.01701 0.01767 0.01836 0.01908 0.01982 0.02061 0.02142 0.02228 0.02319 0.02517 0.02746 0.03029 0.03441 Dynamic Viscosity m, kg/m·s Liquid 6.136 5.054 4.252 3.635 3.149 2.755 2.430 2.158 1.926 1.726 1.551 1.397 1.259 1.195 1.135 1.077 1.022 9.702 9.197 8.710 8.240 7.785 7.343 6.487 5.649 4.790 3.807 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 Vapor Liquid ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 6.820 5.878 5.195 4.686 4.297 3.994 3.755 3.563 3.395 3.266 3.158 3.069 2.996 2.964 2.935 2.909 2.886 2.866 2.850 2.837 2.828 2.824 2.826 2.784 2.834 3.251 4.465 4.372 4.625 4.881 5.143 5.409 5.680 5.956 6.239 6.529 6.827 7.136 7.457 7.794 7.970 8.151 8.339 8.534 8.738 8.952 9.178 9.417 9.674 9.950 1.058 1.138 1.249 1.448 Volume Expansion Coefficient Surface Tension, b, l/K Vapor Liquid N/m Prandtl Number Pr 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 0.827 0.822 0.819 0.817 0.817 0.818 0.821 0.825 0.831 0.839 0.848 0.860 0.875 0.883 0.893 0.905 0.918 0.933 0.950 0.971 0.995 1.025 1.061 1.164 1.343 1.722 3.047 0.00153 0.00157 0.00161 0.00166 0.00171 0.00177 0.00184 0.00192 0.00201 0.00213 0.00226 0.00242 0.00262 0.00273 0.00286 0.00301 0.00318 0.00337 0.00358 0.00384 0.00413 0.00448 0.00491 0.00609 0.00811 0.01248 0.02847 0.02630 0.02486 0.02344 0.02202 0.02062 0.01923 0.01785 0.01649 0.01515 0.01382 0.01251 0.01122 0.00996 0.00934 0.00872 0.00811 0.00751 0.00691 0.00633 0.00575 0.00518 0.00463 0.00408 0.00303 0.00204 0.00114 0.00037 Note 1: Kinematic viscosity n and thermal diffusivity a can be calculated from their definitions, n ⫽ m/r and a ⫽ k/mcp ⫽ n/Pr. The properties listed here (except the vapor density) can be used at any pressures with negligible error except at temperatures near the critical-point value. Note 2: The unit kJ/kg·⬚C for specific heat is equivalent to kJ/kg·K, and the unit W/m·⬚C for thermal conductivity is equivalent to W/m·K. Source: Data generated from the EES software developed by S. A. Klein and F. L. Alvarado. Original sources: Reiner Tillner-Roth, “Fundamental Equations of State,” Shaker, Verlag, Aachan, 1998; B. A. Younglove and J. F. Ely, “Thermophysical Properties of Fluids. II Methane, Ethane, Propane, Isobutane, and Normal Butane,” J. Phys. Chem. Ref. Data, Vol. 16, No. 4, 1987; G.R. Somayajulu, “A Generalized Equation for Surface Tension from the Triple-Point to the CriticalPoint,” International Journal of Thermophysics, Vol. 9, No. 4, 1988. cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 882 882 APPENDIX 1 TABLE A–13 Properties of liquids Temp. T, ⬚C Density r, kg/m3 Specific Heat cp, J/kg·K Thermal Conductivity k, W/m·K Thermal Diffusivity a, m2/s Dynamic Viscosity m, kg/m·s Kinematic Viscosity n, m2/s Prandtl Number Pr Volume Expansion Coeff. b, 1/K Methane [CH4] ⫺160 ⫺150 ⫺140 ⫺130 ⫺120 ⫺110 ⫺100 ⫺90 420.2 405.0 388.8 371.1 351.4 328.8 301.0 261.7 3492 3580 3700 3875 4146 4611 5578 8902 0.1863 0.1703 0.1550 0.1402 0.1258 0.1115 0.0967 0.0797 1.270 1.174 1.077 9.749 8.634 7.356 5.761 3.423 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺7 10⫺7 10⫺7 10⫺8 10⫺8 10⫺8 10⫺8 10⫺8 1.133 9.169 7.551 6.288 5.257 4.377 3.577 2.761 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺4 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 2.699 2.264 1.942 1.694 1.496 1.331 1.188 1.055 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺7 10⫺7 10⫺7 10⫺7 10⫺7 10⫺7 10⫺7 10⫺7 2.126 1.927 1.803 1.738 1.732 1.810 2.063 3.082 0.00352 0.00391 0.00444 0.00520 0.00637 0.00841 0.01282 0.02922 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 7.429 6.531 5.795 5.185 4.677 4.250 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺7 10⫺7 10⫺7 10⫺7 10⫺7 10⫺7 7.414 6.622 5.980 5.453 5.018 4.655 0.00118 0.00120 0.00123 0.00127 0.00132 0.00137 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺5 10⫺5 1.360 8.531 5.942 4.420 3.432 2.743 2.233 1.836 1.509 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺7 10⫺7 10⫺7 10⫺7 10⫺7 10⫺7 10⫺7 10⫺7 12.65 8.167 6.079 4.963 4.304 3.880 3.582 3.363 3.256 0.00142 0.00150 0.00161 0.00177 0.00199 0.00232 0.00286 0.00385 0.00628 8.219 5.287 3.339 1.970 1.201 7.878 5.232 3.464 2.455 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺4 10⫺4 10⫺4 10⫺4 84,101 54,327 34,561 20,570 12,671 8,392 5,631 3,767 2,697 4.242 9.429 2.485 8.565 3.794 2.046 1.241 8.029 6.595 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺3 10⫺4 10⫺4 10⫺5 10⫺5 10⫺5 10⫺5 10⫺6 10⫺6 46,636 10,863 2,962 1,080 499.3 279.1 176.3 118.1 98.31 Methanol [CH3(OH)] 20 30 40 50 60 70 788.4 779.1 769.6 760.1 750.4 740.4 2515 2577 2644 2718 2798 2885 0.1987 0.1980 0.1972 0.1965 0.1957 0.1950 1.002 9.862 9.690 9.509 9.320 9.128 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫺100 ⫺75 ⫺50 ⫺25 0 25 50 75 100 683.8 659.3 634.3 608.2 580.6 550.7 517.3 478.5 429.6 1881 1970 2069 2180 2306 2455 2640 2896 3361 0.1383 0.1357 0.1283 0.1181 0.1068 0.0956 0.0851 0.0757 0.0669 1.075 1.044 9.773 8.906 7.974 7.069 6.233 5.460 4.634 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺7 10⫺7 10⫺8 10⫺8 10⫺8 10⫺8 10⫺8 10⫺8 10⫺8 ⫺8 10⫺7 10⫺8 10⫺8 10⫺8 10⫺8 10⫺8 5.857 5.088 4.460 3.942 3.510 3.146 Isobutane (R600a) 9.305 5.624 3.769 2.688 1.993 1.510 1.155 8.785 6.483 Glycerin 0 5 10 15 20 25 30 35 40 1276 1273 1270 1267 1264 1261 1258 1255 1252 2262 2288 2320 2354 2386 2416 2447 2478 2513 0.2820 0.2835 0.2846 0.2856 0.2860 0.2860 0.2860 0.2860 0.2863 9.773 9.732 9.662 9.576 9.484 9.388 9.291 9.195 9.101 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 1797 1881 1964 2048 2132 2220 2308 2395 2441 0.1469 0.1450 0.1444 0.1404 0.1380 0.1367 0.1347 0.1330 0.1327 9.097 8.680 8.391 7.934 7.599 7.330 7.042 6.798 6.708 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10 10⫺8 10⫺8 10⫺8 10⫺8 10⫺8 10⫺8 10⫺8 10⫺8 10.49 6.730 4.241 2.496 1.519 0.9934 0.6582 0.4347 0.3073 Engine Oil (unused) 0 20 40 60 80 100 120 140 150 899.0 888.1 876.0 863.9 852.0 840.0 828.9 816.8 810.3 10⫺8 10⫺8 10⫺8 10⫺8 10⫺8 10⫺8 10⫺8 10⫺8 10⫺8 3.814 0.8374 0.2177 0.07399 0.03232 0.01718 0.01029 0.006558 0.005344 Source: Data generated from the EES software developed by S. A. Klein and F. L. Alvarado. Originally based on various sources. 0.00070 0.00070 0.00070 0.00070 0.00070 0.00070 0.00070 0.00070 0.00070 cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 883 883 APPENDIX 1 TABLE A–14 Properties of liquid metals Temp. T, ⬚C Density r, kg/m3 Specific Heat cp, J/kg·K Thermal Conductivity k, W/m·K Thermal Diffusivity a, m2/s Dynamic Viscosity m, kg/m·s Kinematic Viscosity n, m2/s Volume Expansion Coeff. b, 1/K Prandtl Number Pr Mercury (Hg) Melting Point: ⫺39 ⬚C 0 25 50 75 100 150 200 250 300 13595 13534 13473 13412 13351 13231 13112 12993 12873 140.4 139.4 138.6 137.8 137.1 136.1 135.5 135.3 135.3 8.18200 8.51533 8.83632 9.15632 9.46706 10.07780 10.65465 11.18150 11.68150 4.287 4.514 4.734 4.956 5.170 5.595 5.996 6.363 6.705 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 1.687 1.534 1.423 1.316 1.245 1.126 1.043 9.820 9.336 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 1.241 1.133 1.056 9.819 9.326 8.514 7.959 7.558 7.252 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺7 10⫺7 10⫺7 10⫺8 10⫺8 10⫺8 10⫺8 10⫺8 10⫺8 0.0289 0.0251 0.0223 0.0198 0.0180 0.0152 0.0133 0.0119 0.0108 10⫺3 10⫺3 10⫺3 10⫺3 10⫺4 1.545 1.436 1.215 1.048 9.157 ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺7 10⫺7 10⫺7 10⫺7 10⫺8 0.01381 0.01310 0.01154 0.01022 0.00906 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 2.167 1.976 1.814 1.702 1.589 1.475 1.360 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺7 10⫺7 10⫺7 10⫺7 10⫺7 10⫺7 10⫺7 0.02252 0.02048 0.01879 0.01771 0.01661 0.01549 0.01434 7.432 5.967 4.418 3.188 2.909 2.614 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺7 10⫺7 10⫺7 10⫺7 10⫺7 10⫺7 0.01106 0.008987 0.006751 0.004953 0.004593 0.004202 4.213 3.456 2.652 2.304 2.126 ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺7 10⫺7 10⫺7 10⫺7 10⫺7 0.006023 0.004906 0.00374 0.003309 0.003143 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺7 10⫺7 10⫺7 10⫺7 10⫺7 10⫺7 0.02102 0.01611 0.01161 0.00753 0.00665 0.00579 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺3 10⫺4 10⫺4 1.810 1.810 1.810 1.810 1.810 1.810 1.815 1.829 1.854 Bismuth (Bi) Melting Point: 271⬚C 350 400 500 600 700 9969 9908 9785 9663 9540 400 450 500 550 600 650 700 10506 10449 10390 10329 10267 10206 10145 146.0 148.2 152.8 157.3 161.8 16.28 16.10 15.74 15.60 15.60 158 156 155 155 155 155 155 15.97 15.74 15.54 15.39 15.23 15.07 14.91 1.118 1.096 1.052 1.026 1.010 ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 1.540 1.422 1.188 1.013 8.736 ⫻ ⫻ ⫻ ⫻ ⫻ Lead (Pb) Melting Point: 327⬚C 9.623 9.649 9.651 9.610 9.568 9.526 9.483 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 10⫺6 2.277 2.065 1.884 1.758 1.632 1.505 1.379 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ Sodium (Na) Melting Point: 98⬚C 100 200 300 400 500 600 927.3 902.5 877.8 853.0 828.5 804.0 1378 1349 1320 1296 1284 1272 85.84 80.84 75.84 71.20 67.41 63.63 6.718 6.639 6.544 6.437 6.335 6.220 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 6.892 5.385 3.878 2.720 2.411 2.101 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 Potassium (K) Melting Point: 64⬚C 200 300 400 500 600 795.2 771.6 748.0 723.9 699.6 790.8 772.8 754.8 750.0 750.0 43.99 42.01 40.03 37.81 35.50 6.995 7.045 7.090 6.964 6.765 ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 3.350 2.667 1.984 1.668 1.487 ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 Sodium-Potassium (%22Na-%78K) Melting Point: ⫺11⬚C 100 200 300 400 500 600 847.3 823.2 799.1 775.0 751.5 728.0 944.4 922.5 900.6 879.0 880.1 881.2 25.64 26.27 26.89 27.50 27.89 28.28 3.205 3.459 3.736 4.037 4.217 4.408 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 5.707 4.587 3.467 2.357 2.108 1.859 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 6.736 5.572 4.339 3.041 2.805 2.553 Source: Data generated from the EES software developed by S. A. Klein and F. L. Alvarado. Originally based on various sources. ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 884 884 APPENDIX 1 TABLE A–15 Properties of air at 1 atm pressure Temp. T, ⬚C Density r, kg/m3 Specific Heat cp, J/kg·K Thermal Conductivity k, W/m·K ⫺150 ⫺100 ⫺50 ⫺40 ⫺30 ⫺20 ⫺10 0 5 10 15 20 25 30 35 40 45 50 60 70 80 90 100 120 140 160 180 200 250 300 350 400 450 500 600 700 800 900 1000 1500 2000 2.866 2.038 1.582 1.514 1.451 1.394 1.341 1.292 1.269 1.246 1.225 1.204 1.184 1.164 1.145 1.127 1.109 1.092 1.059 1.028 0.9994 0.9718 0.9458 0.8977 0.8542 0.8148 0.7788 0.7459 0.6746 0.6158 0.5664 0.5243 0.4880 0.4565 0.4042 0.3627 0.3289 0.3008 0.2772 0.1990 0.1553 983 966 999 1002 1004 1005 1006 1006 1006 1006 1007 1007 1007 1007 1007 1007 1007 1007 1007 1007 1008 1008 1009 1011 1013 1016 1019 1023 1033 1044 1056 1069 1081 1093 1115 1135 1153 1169 1184 1234 1264 0.01171 0.01582 0.01979 0.02057 0.02134 0.02211 0.02288 0.02364 0.02401 0.02439 0.02476 0.02514 0.02551 0.02588 0.02625 0.02662 0.02699 0.02735 0.02808 0.02881 0.02953 0.03024 0.03095 0.03235 0.03374 0.03511 0.03646 0.03779 0.04104 0.04418 0.04721 0.05015 0.05298 0.05572 0.06093 0.06581 0.07037 0.07465 0.07868 0.09599 0.11113 Thermal Diffusivity a, m2/s 4.158 8.036 1.252 1.356 1.465 1.578 1.696 1.818 1.880 1.944 2.009 2.074 2.141 2.208 2.277 2.346 2.416 2.487 2.632 2.780 2.931 3.086 3.243 3.565 3.898 4.241 4.593 4.954 5.890 6.871 7.892 8.951 1.004 1.117 1.352 1.598 1.855 2.122 2.398 3.908 5.664 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺6 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 Dynamic Viscosity m, kg/m·s 8.636 1.189 1.474 1.527 1.579 1.630 1.680 1.729 1.754 1.778 1.802 1.825 1.849 1.872 1.895 1.918 1.941 1.963 2.008 2.052 2.096 2.139 2.181 2.264 2.345 2.420 2.504 2.577 2.760 2.934 3.101 3.261 3.415 3.563 3.846 4.111 4.362 4.600 4.826 5.817 6.630 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 Kinematic Viscosity n, m2/s 3.013 5.837 9.319 1.008 1.087 1.169 1.252 1.338 1.382 1.426 1.470 1.516 1.562 1.608 1.655 1.702 1.750 1.798 1.896 1.995 2.097 2.201 2.306 2.522 2.745 2.975 3.212 3.455 4.091 4.765 5.475 6.219 6.997 7.806 9.515 1.133 1.326 1.529 1.741 2.922 4.270 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺6 10⫺6 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 Prandtl Number Pr 0.7246 0.7263 0.7440 0.7436 0.7425 0.7408 0.7387 0.7362 0.7350 0.7336 0.7323 0.7309 0.7296 0.7282 0.7268 0.7255 0.7241 0.7228 0.7202 0.7177 0.7154 0.7132 0.7111 0.7073 0.7041 0.7014 0.6992 0.6974 0.6946 0.6935 0.6937 0.6948 0.6965 0.6986 0.7037 0.7092 0.7149 0.7206 0.7260 0.7478 0.7539 Note: For ideal gases, the properties cp, k, m, and Pr are independent of pressure. The properties r, n, and a at a pressure P (in atm) other than 1 atm are determined by multiplying the values of r at the given temperature by P and by dividing n and a by P. Source: Data generated from the EES software developed by S. A. Klein and F. L. Alvarado. Original sources: Keenan, Chao, Keyes, Gas Tables, Wiley, 1984; and Thermophysical Properties of Matter. Vol. 3: Thermal Conductivity, Y. S. Touloukian, P. E. Liley, S. C. Saxena, Vol. 11: Viscosity, Y. S. Touloukian, S. C. Saxena, and P. Hestermans, IFI/Plenun, NY, 1970, ISBN 0-306067020-8. cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 885 885 APPENDIX 1 TABLE A–16 Properties of gases at 1 atm pressure Temp. T, ⬚C Density r, kg/m3 Specific Heat cp, J/kg·K Thermal Conductivity k, W/m·K Thermal Diffusivity a, m2/s Dynamic Viscosity m, kg/m·s Kinematic Viscosity n, m2/s Prandtl Number Pr Carbon Dioxide, CO2 ⫺50 0 50 100 150 200 300 400 500 1000 1500 2000 2.4035 1.9635 1.6597 1.4373 1.2675 1.1336 0.9358 0.7968 0.6937 0.4213 0.3025 0.2359 746 811 866.6 914.8 957.4 995.2 1060 1112 1156 1292 1356 1387 0.01051 0.01456 0.01858 0.02257 0.02652 0.03044 0.03814 0.04565 0.05293 0.08491 0.10688 0.11522 5.860 9.141 1.291 1.716 2.186 2.698 3.847 5.151 6.600 1.560 2.606 3.521 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 1.129 1.375 1.612 1.841 2.063 2.276 2.682 3.061 3.416 4.898 6.106 7.322 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 4.699 7.003 9.714 1.281 1.627 2.008 2.866 3.842 4.924 1.162 2.019 3.103 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺6 10⫺6 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺4 10⫺4 10⫺4 0.8019 0.7661 0.7520 0.7464 0.7445 0.7442 0.7450 0.7458 0.7460 0.7455 0.7745 0.8815 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺4 10⫺4 10⫺4 10⫺4 1.378 1.629 1.863 2.080 2.283 2.472 2.812 3.111 3.379 4.557 6.321 9.826 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 9.012 1.303 1.764 2.274 2.830 3.426 4.722 6.136 7.653 1.700 3.284 6.543 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺4 10⫺4 10⫺4 0.7840 0.7499 0.7328 0.7239 0.7191 0.7164 0.7134 0.7111 0.7087 0.7080 0.7733 0.9302 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺4 10⫺4 10⫺4 10⫺4 8.564 1.028 1.191 1.345 1.491 1.630 1.886 2.119 2.334 3.281 4.434 6.360 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 9.774 1.436 1.969 2.567 3.227 3.944 5.529 7.297 9.228 2.136 4.022 7.395 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺4 10⫺4 10⫺4 0.8116 0.7494 0.7282 0.7247 0.7284 0.7344 0.7450 0.7501 0.7502 0.7331 0.7936 1.0386 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 10⫺4 7.293 8.391 9.427 1.041 1.136 1.228 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺6 10⫺6 10⫺5 10⫺5 10⫺5 6.624 9.329 1.240 1.582 1.957 2.365 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺5 10⫺5 10⫺4 10⫺4 10⫺4 10⫺4 0.6562 0.7071 0.7191 0.7196 0.7174 0.7155 10⫺6 10⫺6 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺4 10⫺4 10⫺4 Carbon Monoxide, CO ⫺50 0 50 100 150 200 300 400 500 1000 1500 2000 1.5297 1.2497 1.0563 0.9148 0.8067 0.7214 0.5956 0.5071 0.4415 0.2681 0.1925 0.1502 1081 1048 1039 1041 1049 1060 1085 1111 1135 1226 1279 1309 0.01901 0.02278 0.02641 0.02992 0.03330 0.03656 0.04277 0.04860 0.05412 0.07894 0.10458 0.13833 1.149 1.739 2.407 3.142 3.936 4.782 6.619 8.628 1.079 2.401 4.246 7.034 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ Methane, CH4 ⫺50 0 50 100 150 200 300 400 500 1000 1500 2000 0.8761 0.7158 0.6050 0.5240 0.4620 0.4132 0.3411 0.2904 0.2529 0.1536 0.1103 0.0860 2243 2217 2302 2443 2611 2791 3158 3510 3836 5042 5701 6001 0.02367 0.03042 0.03766 0.04534 0.05344 0.06194 0.07996 0.09918 0.11933 0.22562 0.31857 0.36750 1.204 1.917 2.704 3.543 4.431 5.370 7.422 9.727 1.230 2.914 5.068 7.120 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ Hydrogen, H2 ⫺50 0 50 100 150 200 0.11010 0.08995 0.07603 0.06584 0.05806 0.05193 12635 13920 14349 14473 14492 14482 0.1404 0.1652 0.1881 0.2095 0.2296 0.2486 1.009 1.319 1.724 2.199 2.729 3.306 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 886 886 APPENDIX 1 TABLE A–16 Properties of gases at 1 atm pressure (Concluded) Temp. T, ⬚C Density r, kg/m3 300 400 500 1000 1500 2000 0.04287 0.03650 0.03178 0.01930 0.01386 0.01081 Specific Heat cp, J/kg·K 14481 14540 14653 15577 16553 17400 Thermal Conductivity k, W/m·K 0.2843 0.3180 0.3509 0.5206 0.6581 0.5480 Thermal Diffusivity a, m2/s 4.580 5.992 7.535 1.732 2.869 2.914 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ Dynamic Viscosity m, kg/m·s Kinematic Viscosity n, m2/s Prandtl Number Pr 10⫺4 10⫺4 10⫺4 10⫺3 10⫺3 10⫺3 1.403 1.570 1.730 2.455 3.099 3.690 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 3.274 4.302 5.443 1.272 2.237 3.414 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺4 10⫺4 10⫺4 10⫺3 10⫺3 10⫺3 0.7149 0.7179 0.7224 0.7345 0.7795 1.1717 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺4 10⫺4 10⫺4 10⫺4 1.390 1.640 1.874 2.094 2.300 2.494 2.849 3.166 3.451 4.594 5.562 6.426 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 9.091 1.312 1.774 2.289 2.851 3.457 4.783 6.242 7.816 1.713 2.889 4.278 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺4 10⫺4 10⫺4 0.6655 0.7121 0.7114 0.7056 0.7025 0.7025 0.7078 0.7153 0.7215 0.7022 0.5969 0.4483 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺4 10⫺4 10⫺4 10⫺4 1.616 1.916 2.194 2.451 2.694 2.923 3.350 3.744 4.114 5.732 7.133 8.417 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 9.246 1.342 1.818 2.346 2.923 3.546 4.923 6.463 8.156 1.871 3.243 4.907 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺4 10⫺4 10⫺4 0.7694 0.7198 0.7053 0.7019 0.7019 0.7025 0.7030 0.7023 0.7010 0.6986 0.6985 0.6873 7.187 8.956 1.078 1.265 1.456 1.650 2.045 2.446 2.847 4.762 6.411 7.808 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺6 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 7.305 1.114 1.587 2.150 2.806 3.556 5.340 7.498 1.002 2.761 5.177 8.084 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺4 10⫺4 10⫺4 10⫺4 1.0047 1.0033 0.9944 0.9830 0.9712 0.9599 0.9401 0.9240 0.9108 0.8639 0.8233 0.7833 Nitrogen, N2 ⫺50 0 50 100 150 200 300 400 500 1000 1500 2000 1.5299 1.2498 1.0564 0.9149 0.8068 0.7215 0.5956 0.5072 0.4416 0.2681 0.1925 0.1502 957.3 1035 1042 1041 1043 1050 1070 1095 1120 1213 1266 1297 0.02001 0.02384 0.02746 0.03090 0.03416 0.03727 0.04309 0.04848 0.05358 0.07938 0.11793 0.18590 1.366 1.843 2.494 3.244 4.058 4.921 6.758 8.727 1.083 2.440 4.839 9.543 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ Oxygen, O2 ⫺50 0 50 100 150 200 300 400 500 1000 1500 2000 1.7475 1.4277 1.2068 1.0451 0.9216 0.8242 0.6804 0.5793 0.5044 0.3063 0.2199 0.1716 984.4 928.7 921.7 931.8 947.6 964.7 997.1 1025 1048 1121 1165 1201 0.02067 0.02472 0.02867 0.03254 0.03637 0.04014 0.04751 0.05463 0.06148 0.09198 0.11901 0.14705 1.201 1.865 2.577 3.342 4.164 5.048 7.003 9.204 1.163 2.678 4.643 7.139 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ Water Vapor, H2O ⫺50 0 50 100 150 200 300 400 500 1000 1500 2000 0.9839 0.8038 0.6794 0.5884 0.5189 0.4640 0.3831 0.3262 0.2840 0.1725 0.1238 0.0966 1892 1874 1874 1887 1908 1935 1997 2066 2137 2471 2736 2928 0.01353 0.01673 0.02032 0.02429 0.02861 0.03326 0.04345 0.05467 0.06677 0.13623 0.21301 0.29183 7.271 1.110 1.596 2.187 2.890 3.705 5.680 8.114 1.100 3.196 6.288 1.032 ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 10⫺6 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺4 10⫺4 10⫺4 10⫺3 Note: For ideal gases, the properties cp, k, m, and Pr are independent of pressure. The properties r, n, and a at a pressure P (in atm) other than 1 atm are determined by multiplying the values of p at the given temperature by r and by dividing n and a by P. Source: Data generated from the EES software developed by S. A. Klein and F. L. Alvarado. Originally based on various sources. cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 887 887 APPENDIX 1 TABLE A–17 Properties of the atmosphere at high altitude Altitude, z, m Temperature, T , ⬚C Pressure, P, kPa Gravity g, m/s2 Speed of Sound, c, m/s Density, r, kg/m3 0 200 400 600 800 1000 1200 1400 1600 1800 2000 2200 2400 2600 2800 3000 3200 3400 3600 3800 4000 4200 4400 4600 4800 5000 5200 5400 5600 5800 6000 6200 6400 6600 6800 7000 8000 9000 10,000 12,000 14,000 16,000 18,000 15.00 13.70 12.40 11.10 9.80 8.50 7.20 5.90 4.60 3.30 2.00 0.70 ⫺0.59 ⫺1.89 ⫺3.19 ⫺4.49 ⫺5.79 ⫺7.09 ⫺8.39 ⫺9.69 ⫺10.98 ⫺12.3 ⫺13.6 ⫺14.9 ⫺16.2 ⫺17.5 ⫺18.8 ⫺20.1 ⫺21.4 ⫺22.7 ⫺24.0 ⫺25.3 ⫺26.6 ⫺27.9 ⫺29.2 ⫺30.5 ⫺36.9 ⫺43.4 ⫺49.9 ⫺56.5 ⫺56.5 ⫺56.5 ⫺56.5 101.33 98.95 96.61 94.32 92.08 89.88 87.72 85.60 83.53 81.49 79.50 77.55 75.63 73.76 71.92 70.12 68.36 66.63 64.94 63.28 61.66 60.07 58.52 57.00 55.51 54.05 52.62 51.23 49.86 48.52 47.22 45.94 44.69 43.47 42.27 41.11 35.65 30.80 26.50 19.40 14.17 10.53 7.57 9.807 9.806 9.805 9.805 9.804 9.804 9.803 9.802 9.802 9.801 9.800 9.800 9.799 9.799 9.798 9.797 9.797 9.796 9.796 9.795 9.794 9.794 9.793 9.793 9.792 9.791 9.791 9.790 9.789 9.785 9.788 9.788 9.787 9.786 9.785 9.785 9.782 9.779 9.776 9.770 9.764 9.758 9.751 340.3 339.5 338.8 338.0 337.2 336.4 335.7 334.9 334.1 333.3 332.5 331.7 331.0 330.2 329.4 328.6 327.8 327.0 326.2 325.4 324.6 323.8 323.0 322.2 321.4 320.5 319.7 318.9 318.1 317.3 316.5 315.6 314.8 314.0 313.1 312.3 308.1 303.8 299.5 295.1 295.1 295.1 295.1 1.225 1.202 1.179 1.156 1.134 1.112 1.090 1.069 1.048 1.027 1.007 0.987 0.967 0.947 0.928 0.909 0.891 0.872 0.854 0.837 0.819 0.802 0.785 0.769 0.752 0.736 0.721 0.705 0.690 0.675 0.660 0.646 0.631 0.617 0.604 0.590 0.526 0.467 0.414 0.312 0.228 0.166 0.122 Viscosity m, kg/m·s Thermal Conductivity, k, W/m·K ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ ⫻ 0.0253 0.0252 0.0252 0.0251 0.0250 0.0249 0.0248 0.0247 0.0245 0.0244 0.0243 0.0242 0.0241 0.0240 0.0239 0.0238 0.0237 0.0236 0.0235 0.0234 0.0233 0.0232 0.0231 0.0230 0.0229 0.0228 0.0227 0.0226 0.0224 0.0223 0.0222 0.0221 0.0220 0.0219 0.0218 0.0217 0.0212 0.0206 0.0201 0.0195 0.0195 0.0195 0.0195 1.789 1.783 1.777 1.771 1.764 1.758 1.752 1.745 1.739 1.732 1.726 1.720 1.713 1.707 1.700 1.694 1.687 1.681 1.674 1.668 1.661 1.655 1.648 1.642 1.635 1.628 1.622 1.615 1.608 1.602 1.595 1.588 1.582 1.575 1.568 1.561 1.527 1.493 1.458 1.422 1.422 1.422 1.422 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 10⫺5 Source: U.S. Standard Atmosphere Supplements, U.S. Government Printing Office, 1966. Based on year-round mean conditions at 45⬚ latitude and varies with the time of the year and the weather patterns. The conditions at sea level (z ⫽ 0) are taken to be P ⫽ 101.325 kPa, T ⫽ 15⬚C, r ⫽ 1.2250 kg/m3, g ⫽ 9.80665 m2/s. cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 888 888 APPENDIX 1 TABLE A–18 Emissivities of surfaces (a) Metals Material Aluminum Polished Commercial sheet Heavily oxidized Anodized Bismuth, bright Brass Highly polished Polished Dull plate Oxidized Chromium, polished Copper Highly polished Polished Commercial sheet Oxidized Black oxidized Gold Highly polished Bright foil Iron Highly polished Case iron Wrought iron Rusted Oxidized Lead Polished Unoxidized, rough Oxidized Temperature, K Emissivity, e 300–900 400 400–800 300 350 0.04–0.06 0.09 0.20–0.33 0.8 0.34 500–650 350 300–600 450–800 300–1400 0.03–0.04 0.09 0.22 0.6 0.08–0.40 300 300–500 300 600–1000 300 0.02 0.04–0.05 0.15 0.5–0.8 0.78 300–1000 300 0.03–0.06 0.07 300–500 300 300–500 300 500–900 0.05–0.07 0.44 0.28 0.61 0.64–0.78 300–500 300 300 0.06–0.08 0.43 0.63 Material Magnesium, polished Mercury Molybdenum Polished Oxidized Nickel Polished Oxidized Platinum, polished Silver, polished Stainless steel Polished Lightly oxidized Highly oxidized Steel Polished sheet Commercial sheet Heavily oxidized Tin, polished Tungsten Polished Filament Zinc Polished Oxidized Temperature, K Emissivity, e 300–500 300–400 0.07–0.13 0.09–0.12 300–2000 600–800 0.05–0.21 0.80–0.82 500–1200 450–1000 500–1500 300–1000 0.07–0.17 0.37–0.57 0.06–0.18 0.02–0.07 300–1000 600–1000 600–1000 0.17–0.30 0.30–0.40 0.70–0.80 300–500 500–1200 300 300 0.08–0.14 0.20–0.32 0.81 0.05 300–2500 3500 0.03–0.29 0.39 300–800 300 0.02–0.05 0.25 cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 889 889 APPENDIX 1 TABLE A–18 Emissivities of surfaces (Concluded) (b) Nonmetals Material Alumina Aluminum oxide Asbestos Asphalt pavement Brick Common Fireclay Carbon filament Cloth Concrete Glass Window Pyrex Pyroceram Ice Magnesium oxide Masonry Paints Aluminum Black, lacquer, shiny Oils, all colors Red primer White acrylic White enamel Temperature, K Emissivity, e 800–1400 600–1500 300 300 0.65–0.45 0.69–0.41 0.96 0.85–0.93 300 1200 2000 300 300 0.93–0.96 0.75 0.53 0.75–0.90 0.88–0.94 300 300–1200 300–1500 273 400–800 300 0.90–0.95 0.82–0.62 0.85–0.57 0.95–0.99 0.69–0.55 0.80 300 300 300 300 300 300 0.40–0.50 0.88 0.92–0.96 0.93 0.90 0.90 Material Paper, white Plaster, white Porcelain, glazed Quartz, rough, fused Rubber Hard Soft Sand Silicon carbide Skin, human Snow Soil, earth Soot Teflon Water, deep Wood Beech Oak Temperature, K Emissivity, e 300 300 300 300 0.90 0.93 0.92 0.93 300 300 300 600–1500 300 273 300 300–500 300–500 273–373 0.93 0.86 0.90 0.87–0.85 0.95 0.80–0.90 0.93–0.96 0.95 0.85–0.92 0.95–0.96 300 300 0.94 0.90 cen98128_App-A_p865-892.qxd 1/8/10 3:29 PM Page 890 890 APPENDIX 1 TABLE A–19 Solar radiative properties of materials Description/composition Aluminum Polished Anodized Quartz-overcoated Foil Brick, red (Purdue) Concrete Galvanized sheet metal Clean, new Oxidized, weathered Glass, 3.2-mm thickness Float or tempered Low iron oxide type Marble, slightly off-white (nonreflective) Metal, plated Black sulfide Black cobalt oxide Black nickel oxide Black chrome Mylar, 0.13-mm thickness Paints Black (Parsons) White, acrylic White, zinc oxide Paper, white Plexiglas, 3.2-mm thickness Porcelain tiles, white (reflective glazed surface) Roofing tiles, bright red Dry surface Wet surface Sand, dry Off-white Dull red Snow Fine particles, fresh Ice granules Steel Mirror-finish Heavily rusted Stone (light pink) Tedlar, 0.10-mm thickness Teflon, 0.13-mm thickness Wood Solar Absorptivity, as Emissivity, e, at 300 K Ratio, as /e 0.09 0.14 0.11 0.15 0.63 0.60 0.03 0.84 0.37 0.05 0.93 0.88 3.0 0.17 0.30 3.0 0.68 0.68 0.65 0.80 0.13 0.28 5.0 2.9 Solar Transmissivity, ts 0.79 0.88 0.40 0.88 0.92 0.93 0.92 0.87 0.10 0.30 0.08 0.09 0.45 9.2 3.1 11 9.7 0.87 0.98 0.26 0.16 0.27 0.98 0.90 0.93 0.83 1.0 0.29 0.17 0.32 0.26 0.85 0.30 0.65 0.88 0.85 0.91 0.76 0.96 0.52 0.73 0.82 0.86 0.63 0.82 0.13 0.33 0.82 0.89 0.16 0.37 0.41 0.89 0.65 0.05 0.92 0.87 8.2 0.96 0.74 0.90 0.92 0.92 0.59 0.90 0.66 Source: V. C. Sharma and A. Sharma, “Solar Properties of Some Building Elements,” Energy 14 (1989), pp. 805–810, and other sources. 0.1 4 5 6 8 104 2(104) 3 4 5 6 0 0.9–9 0.5 0.01 0.0015 0.26 0.15 0.046 0.002 0.045 mm Roughness, e 2(105) 3 4 5 6 Reynolds number, Re 8 105 Smooth pipes e/D = 0 2(106) 3 4 5 6 e/D = 0.000001 8 106 Fully rough turbulent flow (f levels off) flow are evaluated from the Colebrook equation 冢 冣 /D 2.51 1 ⫽⫺2log10 ⫹ . 3.7 Re 兹f 兹f 2(107) 3 4 5 6 0.00001 8 108 0.00005 0.0001 0.0002 0.0004 0.001 0.0008 0.0006 0.002 0.004 0.006 0.01 0.008 0.015 0.02 0.03 0.04 0.05 Relative roughness, e/D 2 L rV . Friction factors in the turbulent D 2 8 107 e/D = 0.000005 The Moody chart for the friction factor for fully developed flow in circular pipes for use in the head loss relation ⌬PL⫽ f 2(103) 3 0 0.003–0.03 0.0016 0.000033 0.000005 0.00085 0.0005 0.00015 0.000007 0.00015 Glass, plastic Concrete Wood stave Rubber, smoothed Copper or brass tubing Cast iron Galvanized iron Wrought iron Stainless steel Commercial steel 103 ft FIGURE A–20 0.008 0.009 i Material e 0.01 64/R 0.015 ,f= low nar f 0.02 Transitional Turbulent flow flow 3:29 PM 0.025 0.03 Laminar flow Lam 0.04 0.05 0.06 0.07 0.08 0.09 1/8/10 Darcy friction factor, f cen98128_App-A_p865-892.qxd Page 891 891 APPENDIX 1