Final Exam for Organic II 200pts(Weighted as 300) Name Good luck
advertisement
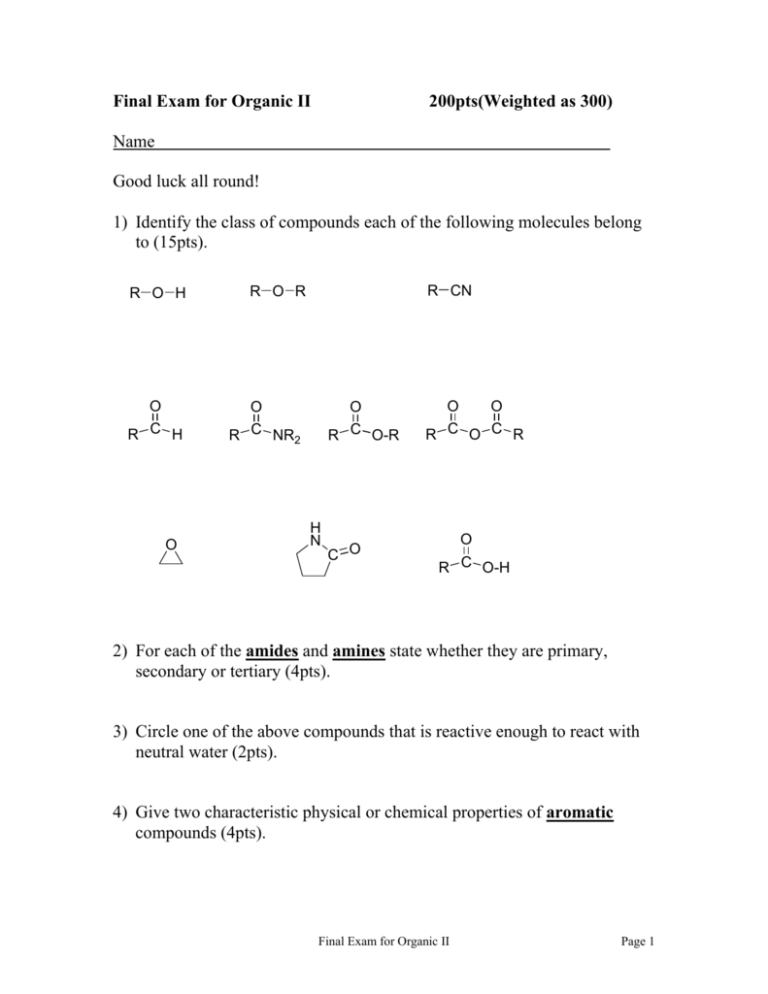
Final Exam for Organic II 200pts(Weighted as 300) Name Good luck all round! 1) Identify the class of compounds each of the following molecules belong to (15pts). R O H O R C H O R CN R O R O R C NR2 O R C O-R H N C O O O R C O C R O R C O-H 2) For each of the amides and amines state whether they are primary, secondary or tertiary (4pts). 3) Circle one of the above compounds that is reactive enough to react with neutral water (2pts). 4) Give two characteristic physical or chemical properties of aromatic compounds (4pts). Final Exam for Organic II Page 1 5) Indicate which of the following molecules are aromatic, non-aromatic or anti-aromatic. (Assume all the molecules are planar). (15pts) O H3C H3C CH3 CH3 H3C CH3 H H N+ + O NO2 S N N N + NO2 6) Pick one of the above antiaromatic molecules, and use the polygon rule to demonstrate its antiaromaticity. (8pts) Final Exam for Organic II Page 2 7) Give the products in six of the following reactions, paying attention to regio/stereochemistry where applicable. (18pts) heat NC CN O CH2CH2CH3 Ph C CH2 Br2, H2O Excess HBr KOH A B Ph Excess HI OH O F CO, HCl CuCl, AlCl3 CN O heat CH3 Br2, FeBr3 NHCOCH3 Final Exam for Organic II Page 3 8) The below heterocycle contains two Nitrogen atoms, and is 6π Hückel aromatic. CH3 N N The lone pairs on the two Nitrogens play different roles in the aromaticity of the molecule, and consequently one Nitrogen will get protonated before the other. Describe which one gets protonated first, and explain their differing basicity. (10pts) Final Exam for Organic II Page 4 9) Give reagents and conditions to accomplish five of the following transformations. (15pts) CH3 CH3 O CH2-OH CH2-O-CH2 O2N O2N NO2 HO H O H3C H3C H Ph Ph Ph Ph CH2CH3 CH3CH2 CH2CH2CH3 CO2H CH2CH3 HO2C O CO2H Br A O CH2CH2CH3 (no marks for A)!!! Final Exam for Organic II Page 5 10) The addition of (1 equivalent of) HBr to cis-1,2-dimethyl-3,5cyclohexadiene generates a mixture of products. H3C H3C HBr mixture Draw the products, predict their approximate ratio, and mechanistically account for the mixture of products. (10pts) Final Exam for Organic II Page 6 11) Circle the stronger base in the following pairs, and in a sentence explain your choice. (6pts) (a) O2N NH2 NH2 O (b) F3C N H C CH3 H3C N H H2 C CF3 12) Circle the stronger acid in the following pairs, and in a sentence explain your answer. (12pts) (a) O H3C C OH CO2H CF3CH2 OH CO2H (b) CN O (c) O Cl3C C OH Br3C C OH O O (d) H C OH H3C C OH Final Exam for Organic II Page 7 13) Name five of the following compounds in IUPAC acceptable terms. (15pts) O O O O O O Cl O NH O 14) O Rank methanal (formaldehyde), propanone and propanal in increasing reactivity with nucleophiles, and explain their differing reactivities. (12pts) Final Exam for Organic II Page 8 15) Fill in the blanks for five of the following reactions. (25pts) Br 1) excess Mg ? ? (a) Br 2) excess CO2 + 3) H3O NH2 1) NaNO2, HCl ? ? (b) 2) H3PO2, heat CH2-OCH3 (c) ? NaOCH3 ? CH2-OCH3 D CH2CO2H CH2CONH2 P2O5 ? (d) CH2 CH2CN O C Ph ? (e) CH2CH2Ph ? O C Cl (f) ? ? SOCl2 O C ? Final Exam for Organic II H Page 9 16) Give the mechanism for one of the below conversions (10pts) 1) BuLi S H S H O 2) CH3CH2Br 3) H3O+, HgCl2 CH3CH2 C CH2CH3 or CH3CH2CH2CH2Cl, AlCl3 Final Exam for Organic II Page 10 17) Give the starting material and mechanism for one of the following schemes. (19pts) (a) Cl2, KOH NH2 H2O O (b) O Na, NH3 H C2H5OH H (c) 1) CH3Br, Ph3P 2) BuLi 3) Warm Ph C CH2 Ph Final Exam for Organic II Page 11 *Bonus question* (1pt for within $5k, 2 pts within $1k) What is the base rate 10 month salary (i.e. excluding summer compensation and consultancy, etc) for an assistant chemistry professor at Rutgers? Final Exam for Organic II Page 12