Sample Page 1
advertisement

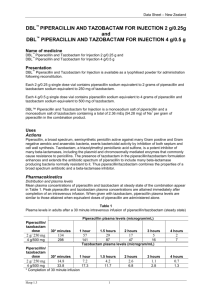
PIPERACILLIN AND TAZOBACTAM count, cholestatic jaundice, circulatory arrest, Clostridium difficile associated diarrhea, confusion, convulsions, decreased hematocrit, decreased hemoglobin, decreased serum albumin, depression, dysgeusia, dysuria, electrolyte disturbance, eosinophilia, epistaxis, erythema multiforme, gastritis, genital pruritus, hallucination, hematuria, hemolytic anemia, hemorrhage, hepatitis, hiccups, hyperglycemia, hypersensitivity reaction, hypoglycemia, increased blood glucose, increased blood urea nitrogen, increased gamma-glutamyl transferase, increased serum alkaline phosphatase, increased serum ALT, increased serum AST, increased serum bilirubin, increased serum creatinine, increased thirst, inflammation, interstitial nephritis, intestinal obstruction, leukopenia, leukorrhea, melena, mesenteric embolism, myalgia, myocardial infarction, neutropenia, oliguria, pancytopenia, photophobia, positive direct Coombs test, prolonged partial thromboplastin time, prolonged prothrombin time, pulmonary edema, pulmonary embolism, purpura, renal failure, rigors, Stevens-Johnson syndrome, supraventricular tachycardia, syncope, thrombocythemia, thrombocytopenia, thrombophlebitis, toxic epidermal necrolysis, urinary incontinence, urinary retention, vaginitis, ventricular fibrillation, ventricular tachycardia Drug Interactions Metabolism/Transport Effects None known. Avoid Concomitant Use Avoid concomitant use of Piperacillin and Tazobactam with any of the following: BCG; BCG (Intravesical); Probenecid Increased Effect/Toxicity Piperacillin and Tazobactam may increase the levels/ effects of: Flucloxacillin [Floxacillin]; Methotrexate; Vancomycin; Vecuronium; Vitamin K Antagonists The levels/effects of Piperacillin and Tazobactam may be increased by: Probenecid Decreased Effect Piperacillin and Tazobactam may decrease the levels/ effects of: Aminoglycosides; BCG; BCG (Intravesical); BCG Vaccine (Immunization); Mycophenolate; Sodium Picosulfate; Typhoid Vaccine The levels/effects of Piperacillin and Tazobactam may be decreased by: Tetracycline Derivatives Storage/Stability Vials: Store at 20°C to 25°C (68°F to 77°F) prior to reconstitution. Use single-dose or bulk vials immediately after reconstitution. Discard any unused portion after 24 hours if stored at 20°C to 25°C (68°F to 77°F) or after 48 hours if stored refrigerated (2°C to 8°C [36°F to 46°F]). Do not freeze vials after reconstitution. Stability in IV bags has been demonstrated for up to 24 hours at room temperature and up to 1 week at refrigerated temperature. Stability in an ambulatory IV infusion pump has been demonstrated for a period of 12 hours at room temperature. Galaxy containers: Store at or below -20°C (-4°F). The thawed solution is stable for 14 days under refrigeration (2°C to 8°C [36°F to 46°F]) or 24 hours at 20°C to 25°C (68°F to 77°F). Do not refreeze. Mechanism of Action Piperacillin inhibits bacterial cell wall synthesis by binding to one or more of the penicillinbinding proteins (PBPs); which in turn inhibits the final transpeptidation step of peptidoglycan synthesis in bacterial cell walls, thus inhibiting cell wall biosynthesis. Bacteria eventually lyse due to ongoing activity of cell wall autolytic enzymes (autolysins and murein hydrolases) while cell wall assembly is arrested. Piperacillin exhibits timedependent killing. Tazobactam inhibits many beta-lactamases, including staphylococcal penicillinase and Richmond-Sykes types 2, 3, 4, and 5, including extended 1707 spectrum enzymes; it has only limited activity against class 1 beta-lactamases other than class 1C types. Pharmacokinetics (Adult data unless noted) Both AUC and peak concentrations are dose proportional Distribution: Widely distributed into tissues and body fluids including lungs, intestinal mucosa, female reproductive tissues, interstitial fluid, gallbladder, and bile; penetration into CSF is poor when meninges are uninflamed Vd: Children and Adults: 0.243 L/kg Protein binding: Piperacillin: ~26% to 33% Tazobactam: 31% to 32% Metabolism: Piperacillin: 6% to 9% to desethyl metabolite (weak activity) Tazobactam: ~22% to inactive metabolite Bioavailability: IM: Piperacillin: 71% Tazobactam: 84% Half-life (Pediatric data: Reed 1994): Piperacillin: Infants 2 to 5 months: 1.4 hours Children 6 to 23 months: 0.9 hour Children 2 to 12 years: 0.7 hour Adults: 0.7 to 1.2 hours Metabolite: 1 to 1.5 hours Tazobactam: Infants 2 to 5 months: 1.6 hours Children 6 to 23 months: 1 hour Children 2 to 12 years: 0.8-0.9 hour Adults: 0.7 to 0.9 hour Elimination: Piperacillin and tazobactam are both eliminated by renal tubular secretion and glomerular filtration. Piperacillin, tazobactam, and desethylpiperacillin are also secreted into bile. Piperacillin: 50% to 70% eliminated unchanged in urine Tazobactam: Found in urine at 24 hours, with 20% as the inactive metabolite and 80% as unchanged drug Clearance: Children 9 months to 12 years: 5.64 mL/ minute/kg Dosing: Neonatal Note: Zosyn (piperacillin and tazobactam) is a combination product; each 3.375 g vial contains 3 g piperacillin sodium and 0.375 g tazobactam sodium in an 8:1 ratio. Dosage recommendations are based on the piperacillin component. General dosing, susceptible infection: Weight-directed dosing (Red Book, 2012): IV: Body weight <1 kg: PNA ≤14 days: 100 mg piperacillin/kg/dose every 12 hours PNA 15 to 28 days: 100 mg piperacillin/kg/dose every 8 hours Body weight ≥1 kg: PNA ≤7 days: 100 mg piperacillin/kg/dose every 12 hours PNA 8 to 28 days: 100 mg piperacillin/kg/dose every 8 hours Age-directed dosing: Note: Limited data available, positive efficacy results, dosage regimens variable, dosing partially extrapolated from piperacillin data (Kacet 1992) GA <36 weeks: PNA 0 to 7 days: 75 mg piperacillin/kg/dose every 12 hours PNA 8 to 28 days: 75 mg piperacillin/kg/dose every 8 hours Meningitis: PNA 0 to 7 days: 200 mg piperacillin/kg/ dose every 12 hours was used in two neonates (GA: 31, 35 weeks) (Placzek 1983) GA ≥36 weeks: PNA 0 to 7 days: 75 mg piperacillin/kg/dose every 8 hours PNA 8 to 28 days: 75 mg piperacillin/kg/dose every 6 hours PIPERACILLIN AND TAZOBACTAM Dosing: Usual Pediatric: Note: Zosyn (piperacillin and tazobactam) is a combination product; each 3.375 g vial contains 3 g piperacillin sodium and 0.375 g tazobactam sodium in an 8:1 ratio. Dosage recommendations are based on the piperacillin component. Note: Some centers divide doses every 6 hours for enhanced pharmacodynamic profile. Unless otherwise specified, dosing presented is based on traditional infusion method (IV infusion over 30 minutes). Dosing is presented in mg/kg/dose and mg/kg/ day; use precaution. General dosing, susceptible infection (Red Book [AAP] 2012): IV: Severe infection: Infants <2 months: 100 mg piperacillin/kg/dose every 6 hours (Bradley 2014) Infants 2 to 9 months: 80 mg piperacillin/kg/dose every 8 hours Infants >9 months, Children, and Adolescents: 100 mg piperacillin/kg/dose every 8 hours; maximum daily dose: 16 g piperacillin/day Appendicitis and/or peritonitis: IV: Infants 2 to 9 months: 80 mg of piperacillin/kg/dose every 8 hours Infants >9 months and Children weighing ≤40 kg: 100 mg piperacillin/kg/dose every 8 hours; maximum dose: 3,000 mg piperacillin/dose Children weighing >40 kg and Adolescents: 3,000 mg piperacillin every 6 hours; maximum daily dose: 16 g piperacillin/day Cystic fibrosis, pseudomonal lung infection: Infants, Children, and Adolescents: Note: Multiple dosing approaches have been evaluated; optimal dose may vary based on disease severity, susceptibility patterns (eg, MIC), or patient tolerability: Standard dosing range: IV: 240 to 400 mg piperacillin/ kg/day divided every 8 hours (Kliegman 2011); others have used 350 to 400 mg/kg/day divided every 4 hours in early piperacillin trials (Zobell 2013) High-dose: Limited data available: IV: 450 mg piperacillin/kg/day every 4 to 6 hours or 600 mg piperacillin/ kg/day divided every 4 hours has been described from early studies of piperacillin alone; usual maximum daily dose: 18 to 24 g piperacillin/day. Note: Piperacillin doses >600 mg/kg/day or an extended duration of therapy (>14 days) have been associated with dose-related adverse effects including serum sickness, immune-mediated hemolytic anemia and bone marrow suppression (Zobell 2013). Intra-abdominal infection, complicated: Infants, Children, and Adolescents: IV: 200 to 300 mg piperacillin/ kg/day divided every 6 to 8 hours; maximum daily dose: 12 g piperacillin/day (Solomkin 2010) Surgical antimicrobial prophylaxis (Bratzler, 2013): IV: Infants 2 to 9 months: 80 mg piperacillin/kg 30 to 60 minutes prior to procedure; may repeat in 2 hours Infants >9 months, Children, and Adolescents weighing ≤40 kg: 100 mg piperacillin/kg 30 to 60 minutes prior to procedure; may repeat in 2 hours. Maximum dose: 3,000 mg piperacillin/dose Adolescents weighing >40 kg: 3000 mg piperacillin 30 to 60 minutes prior to procedure; may repeat in 2 hours Extended-infusion method: Limited data available: Children and Adolescents: IV: 100 mg piperacillin/kg/ dose infused over 4 hours 3 times daily. Dosing based on a prospective, observational study (n=332) in a single children's hospital comparing the extended interval method to traditional dosing (Nichols 2012). Adult: Note: Dosing expressed as total dose of combination product (piperacillin/tazobactam); dosing presented is based on traditional infusion method (IV infusion over 30 minutes) unless otherwise specified as the extended infusion method (IV infusion over 4 hours) 1708 General dosing, susceptible infection: IV: 3.375 g (3,000 mg piperacillin/375 mg tazobactam) every 6 hours or 4.5 g (4,000 mg piperacillin/500 mg tazobactam) every 6 to 8 hours; maximum: 16 g piperacillin/day Diverticulitis, intra-abdominal abscess, peritonitis: IV: 3.375 g every 6 hours; Note: Some clinicians use 4.5 g every 8 hours for empiric coverage since the % time>MIC is similar between the regimens for most pathogens; however, this regimen is not recommended for nosocomial pneumonia or Pseudomonas coverage. Pneumonia, nosocomial: IV: 4.5 g every 6 hours for 7 to 14 days (when used empirically, combination with an aminoglycoside or antipseudomonal fluoroquinolone is recommended; consider discontinuation of additional agent if P. aeruginosa is not isolated) Skin and soft tissue infection: IV: 3.375 g every 6 to 8 hours for 7 to 14 days. Notes: When used for necrotizing infection of skin, fascia, or muscle, combination with clindamycin and ciprofloxacin is recommended (Stevens 2005); for severe diabetic foot infections, recommended treatment duration is up to 4 weeks depending on severity of infection and response to therapy (Lipsky, 2012). Dosing adjustment in renal impairment: Infants, Children, and Adolescents: There are no dosage adjustments provided in the manufacturer's labeling; however, the following have been used by some clinicians (Aronoff 2007): Note: Dosage recommendations are based on the piperacillin component. Dosing based on a usual dose of 200 to 300 mg piperacillin kg/day in divided doses every 6 hours. GFR >50 mL/minute/1.73 m2: No adjustment required GFR 30 to 50 mL/minute/1.73 m2: 35 to 50 mg piperacillin/kg/dose every 6 hours GFR <30 mL/minute/1.73 m2: 35 to 50 mg piperacillin/ kg/dose every 8 hours Intermittent hemodialysis (IHD): Hemodialysis removes 30% to 40% of a piperacillin/tazobactam dose: 50 to 75 mg piperacillin/kg/dose every 12 hours Peritoneal dialysis (PD): Peritoneal dialysis removes 21% of tazobactam and 6% of piperacillin: 50 to 75 mg piperacillin/kg/dose every 12 hours Continuous renal replacement therapy (CRRT): 35 to 50 mg piperacillin kg/dose every 8 hours Adults: Note: Dosing expressed as total dose of combination product (piperacillin/tazobactam). Traditional infusion method (ie, IV infusion over 30 minutes): Manufacturer’s labeling: Nosocomial pneumonia: CrCl >40 mL/minute: No adjustment needed CrCl 20 to 40 mL/minute: 3.375 g every 6 hours CrCl <20 mL/minute: 2.25 g every 6 hours Hemodialysis: 2.25 g every 8 hours with an additional dose of 0.75 g after each dialysis CAPD: Adults: 2.25 g every 8 hours All other indications: CrCl >40 mL/minute: No adjustment needed CrCl 20 to 40 mL/minute: 2.25 g every 6 hours CrCl <20 mL/minute: 2.25 g every 8 hours Hemodialysis: Hemodialysis removes 30% to 40% of a piperacillin/tazobactam dose: 2.25 g every 12 hours with an additional dose of 0.75 g after each dialysis CAPD: Peritoneal dialysis removes 21% of tazobactam and 6% of piperacillin: 2.25 g every 12 hours Dosing adjustment in hepatic impairment: No dosing adjustment required Preparation for Administration Parenteral: Reconstitution: Single-dose vials: Reconstitute with 5 mL of diluent (NS, D5W, or SWFI) per 1,000 mg of piperacillin to yield a PIRBUTEROL concentration of piperacillin 200 mg/mL and tazobactam 25 mg/mL Pharmacy bulk vial: Reconstitute with 152 mL of diluent (NS, D5W or SWFI) to yield a concentration of piperacillin 200 mg/mL and tazobactam 25 mg/mL Intermittent IV infusion: Further dilute dose in a volume of 50 to 150 mL for a usual final concentration of piperacillin 20 to 80 mg/mL; dilution of doses in volumes as low 25 or 37.5 mL have been used in ambulatory infusion pumps Dosage Forms Excipient information presented when available (limited, particularly for generics); consult specific product labeling. Note: 8:1 ratio of piperacillin sodium/tazobactam sodium Infusion [premixed iso-osmotic solution]: Zosyn: 2.25 g: Piperacillin 2 g and tazobactam 0.25 g (50 mL) [contains edetate disodium, sodium 128 mg (5.58 mEq)] Zosyn: 3.375 g: Piperacillin 3 g and tazobactam 0.375 g (50 mL) [contains edetate disodium, sodium 192 mg (8.38 mEq)] Zosyn: 4.5 g: Piperacillin 4 g and tazobactam 0.5 g (100 mL) [contains edetate disodium, sodium 256 mg (11.17 mEq)] Injection, powder for reconstitution: 2.25 g: Piperacillin 2 g and tazobactam 0.25 g; 3.375 g: Piperacillin 3 g and tazobactam 0.375 g; 4.5 g: Piperacillin 4 g and tazobactam 0.5 g; 40.5 g: Piperacillin 36 g and tazobactam 4.5 g Zosyn: 2.25 g: Piperacillin 2 g and tazobactam 0.25 g [contains edetate disodium, sodium 128 mg (5.58 mEq)] Zosyn: 3.375 g: Piperacillin 3 g and tazobactam 0.375 g [contains edetate disodium, sodium 192 mg (8.38 mEq)] Zosyn: 4.5 g: Piperacillin 4 g and tazobactam 0.5 g [contains edetate disodium, sodium 256 mg (11.17 mEq)] Zosyn: 40.5 g: Piperacillin 36 g and tazobactam 4.5 g [contains edetate disodium, sodium 2304 mg (100.4 mEq); bulk pharmacy vial] Administration Parenteral: Intermittent IV infusion: Administer over 30 minutes Extended IV infusion: Administration over 3 to 4 hours has been reported in pediatric and adult patients (Kim 2007; Nichols 2012; Shea 2009). Some penicillins (eg, carbenicillin, ticarcillin, and piperacillin) have been shown to inactivate aminoglycosides in vitro. This has been observed to a greater extent with tobramycin and gentamicin, while amikacin has shown greater stability against inactivation. Concurrent use of these agents may pose a risk of reduced antibacterial efficacy in vivo, particularly in the setting of profound renal impairment. However, definitive clinical evidence is lacking. If combination penicillin/aminoglycoside therapy is desired in a patient with renal dysfunction, separation of doses (if feasible), and routine monitoring of aminoglycoside levels, CBC, and clinical response should be considered. Note: Reformulated Zosyn containing EDTA has been shown to be compatible in vitro for Y-site infusion with amikacin and gentamicin diluted in NS or D5W (applies only to specific concentrations and varies by product; consult manufacturer’s labeling). Reformulated Zosyn containing EDTA is not compatible with tobramycin. Monitoring Parameters Serum electrolytes, bleeding time especially in patients with renal impairment; periodic tests of renal, hepatic, and hematologic function; observe for changes in bowel frequency, CBC with differential; monitor for signs of anaphylaxis during first dose Test Interactions Positive Coombs' [direct] test; false positive reaction for urine glucose using copper-reduction method (Clinitest); may result in false positive results with the Platelia Aspergillus enzyme immunoassay (EIA) Some penicillin derivatives may accelerate the degradation of aminoglycosides in vitro, leading to a potential underestimation of aminoglycoside serum concentration. Note: Reformulated Zosyn containing EDTA has been shown to be compatible in vitro for Y-site infusion with amikacin and gentamicin diluted in NS or D5W (applies only to specific concentrations and varies by product; consult manufacturer’s labeling). Reformulated Zosyn containing EDTA is not compatible with tobramycin. Additional Information Some penicillins (eg, carbenicillin, ticarcillin, and piperacillin) have been shown to inactivate aminoglycosides in vitro. This has been observed to a greater extent with tobramycin and gentamicin, while amikacin has shown greater stability against inactivation. Concurrent use of these agents may pose a risk of reduced antibacterial efficacy in vivo, particularly in the setting of profound renal impairment. However, definitive clinical evidence is lacking. If combination penicillin/aminoglycoside therapy is desired in a patient with renal dysfunction, separation of doses (if feasible), and routine monitoring of aminoglycoside levels, CBC, and clinical response should be considered. Note: Reformulated Zosyn® containing EDTA has been shown to be compatible in vitro for Y-site infusion with amikacin and gentamicin diluted in NS or D5W (applies only to specific concentrations and varies by product; consult manufacturer’s labeling). Reformulated Zosyn® containing EDTA is not compatible with tobramycin. ^ Piperacillin and Tazobactam for Injection (Can) see Piperacillin and Tazobactam on page 1706 ^ Piperacillin and Tazobactam Sodium see Piperacillin and Tazobactam on page 1706 ^ Piperacillin Sodium and Tazobactam Sodium see Piperacillin and Tazobactam on page 1706 Pirbuterol 1709 (peer BYOO ter ole) Brand Names: US Maxair Autohaler [DSC] Therapeutic Category Adrenergic Agonist Agent; Antiasthmatic; Beta 2 -Adrenergic Agonist; Bronchodilator; Sympathomimetic Generic Availability (US) No Use Prevention and treatment of bronchospasm in patients with reversible airway obstruction due to asthma or COPD Pregnancy Risk Factor C Pregnancy Considerations Adverse events have been observed in some animal reproduction studies. Beta-agonists may interfere with uterine contractility if administered during labor. Uncontrolled asthma is associated with adverse events on pregnancy (increased risk of perinatal mortality, preeclampsia, preterm birth, low birth weight infants). Other beta2-receptor agonists are preferred for the treatment of asthma during pregnancy (NAEPP, 2005). Breast-Feeding Considerations It is not known if pirbuterol is excreted into breast milk. The manufacturer recommends that pirbuterol be used in breast-feeding women only if the potential benefit to the mother outweighs the possible risk to the infant. The use of beta2-receptor agonists are not considered a contraindication to breastfeeding (NAEPP, 2005). Contraindications Hypersensitivity to pirbuterol or any component of the formulation Warnings/Precautions Optimize anti-inflammatory treatment before initiating maintenance treatment with pirbuterol. Do not use as a component of chronic therapy without an anti-inflammatory agent. Only the mildest form of asthma (Step 1 and/or exercise-induced) would not