molar heat of fusion of ice
advertisement

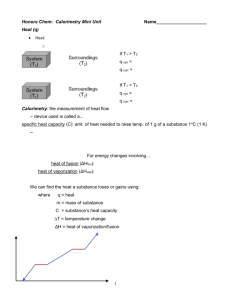
MOLAR HEAT OF FUSION OF ICE INTRODUCTION For a particular substance, the conversion from a solid to a liquid is an endothermic process, whereby energy is absorbed by the system. The conversion from a liquid to a solid is an exothermic process whereby energy is released by the system. For a particular substance, when the state changes from a solidliquid, the direction of energy flow is opposite but the magnitude of the energy change is the same. The molar heat of fusion refers to the quantity of energy released when a substance is converted from a liquid to a solid. THEORY In this experiment we will determine the molar heat of fusion of ice. Your result should approximate the value found on your data table, 6.01kJ/mol. When using a calorimeter, The First Law of Thermodynamics applies. Therefore, the heat lost by the calorimeter will equal the heat gained by the ice. A known quantity of ice will be allowed to melt in a calorimeter. The water in the calorimeter will decrease in temperature as energy is utilized to melt the ice cube. The water that forms from the ice cube will then increase in temperature until the calorimeter water and the water from the ice cube reach the same temperature. In the calculations, we must allow for the heat needed to warm up the ice water that forms from the ice cube. In performing the calculations, we will make a number of assumptions. The first assumption is that the ice is at 0oC. The second is that all of the heat exchanged is with the water contained within the calorimeter. 1 PROCEDURE 1. Use the same balance for all mass determinations. 2. Determine and record the mass of a dry calorimeter apparatus, including the lid, to the nearest 0.01g. Use this mass for all trials. 3. Use a hot plate and a 600 mL beaker, to heat approximately 250 mL of distilled water to approximately 30.0oC. 4. Use a 100 mL graduated cylinder to measure 200.0 mL of the distilled water into the calorimeter. Determine and record the mass of the calorimeter and contents to the nearest 0.01g. 5. Determine and record the initial temperature of the distilled water in the calorimeter to the nearest 0.1oC. 6. Obtain one distilled water ice cube. Place the ice cube on a paper towel and dry it. Place it in the calorimeter. Cover the calorimeter and stir with the thermometer until the ice cube is completely melted. 7. Record the final (lowest) temperature of the water in the calorimeter to the nearest 0.1oC. 8. Determine and record the mass of the calorimeter, the warmed distilled water and the melted ice cube to the nearest 0.1g. 9. Dry all parts of the calorimeter thoroughly. Repeat the above procedure at least three times. Reheat the distilled water used in step 3 again if necessary. OBSERVATIONS Much of what happens in this experiment is not visible as it happens within the calorimeter. Nevertheless, observations are required and you will need to be creative. 2 DATA The following data table may be used by you or may be modified. When recording data, it is important to use the precision identified in the procedure, for all measurements. SAMPLE DATA TABLE TRIAL 1 TRIAL 2 TRIAL 3 a. Mass of calorimeter (to nearest 0.01g) b. Mass of calorimeter and water (to nearest 0.01g) c. Initial temperature of calorimeter (to nearest 0.1oC) d. Final temperature of calorimeter (to nearest 0.1oC) e. Mass of calorimeter, water and melted ice (to nearest 0.01g) CALCULATIONS You will do a set of calculations for each trial above and then average the results. In order to perform the calculations for this experiment, you will need to manipulate the data to isolate the required data. The table below will help with this process. 3 TRIAL 1 TRIAL 2 TRIAL 3 Mass of water in calorimeter (to nearest 0.01g): from data table b-a Mass of ice in calorimeter (to nearest 0.01 g): from data table above e-b Change in temperature of calorimeter water (to nearest 0.1oC): from data table above d-c Change in temperature of the water that forms from the ice cube melting (to nearest 0.1oC): From data table above d-0 The equation for the calculation is the following: nH = m1c1∆t1 – m2c2 ∆t2 where: n= mole of ice m1= mass of water in the calorimeter c1 = specific heat capacity for water ∆t1 = temperature change for the water in the calorimeter m2 = mass of the ice cube c2 = specific heat capacity for water (note this is the water that formed from the ice cube) ∆t2=temperature change for the water that formed from the ice cube 4 CONCLUSION In your conclusion, provide an overview of the lab process and compare your results to the book value for the Molar Heat of Fusion of Ice. REASONS FOR ERROR Indicate any reasons for error that may be associated with this lab and your results. 5