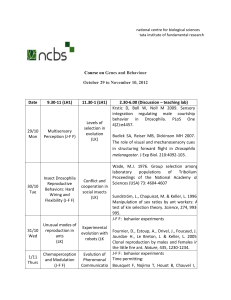
Speciation genes
H Allen Orr, John P Masly and Daven C Presgraves
Until recently, the genes that cause reproductive isolation
remained black boxes. Consequently, evolutionary biologists
were unable to answer several questions about the identities
and characteristics of ‘speciation genes’. Over the past few
years, however, evolutionary geneticists have finally
succeeded in isolating several such genes, providing our first
glimpse at factors that are thought to be representative of
those underlying the origin of species. Evolutionary analysis
of these genes suggests that speciation results from positive
Darwinian selection within species. Molecular evolutionary
study of the genes causing reproductive isolation may
represent an important new phase in the study of speciation.
Addresses
Department of Molecular Biology & Genetics, Cornell University,
Ithaca, New York 14853, USA
e-mail: aorr@mail.rochester.edu
Current Opinion in Genetics & Development 2004, 14:675–679
This review comes from a themed issue on
Genomes and evolution
Edited by David Haig and Steve Henikoff
Available online 5th October 2004
0959-437X/$ – see front matter
# 2004 Elsevier Ltd. All rights reserved.
DOI 10.1016/j.gde.2004.08.009
Abbreviations
HMS hybrid male sterility
NPC nuclear pore complex
Introduction
The founders of the modern synthesis viewed speciation — the evolution of barriers to gene flow between
taxa — as a key issue in evolutionary genetics. The title of
Dobzhansky’s seminal book [1] was, after all, Genetics and
the Origin of Species. Despite this early enthusiasm, our
understanding of the genetics of speciation has lagged far
behind that of most other evolutionary phenomena. In
part, this reflects an obvious methodological problem: the
genetic study of speciation is, almost by definition, the
attempt to do genetics where it cannot be done —
namely, between species.
Until recently, our understanding of speciation was
restricted to comparative and classical genetic patterns.
It was clear, for example, that reproductive isolation
evolves gradually in most taxa (if we ignore polyploid
speciation in plants [2–8]). It was also clear that the
www.sciencedirect.com
‘postzygotic’ form of reproductive isolation — that is,
the sterility or inviability of hybrids — is caused by
epistatic interactions between alleles from different species at two or more loci: although such alleles function
well on their normal within-species genetic background,
they fail to interact properly in a hybrid genome [9,10].
Evolutionary biologists were not, however, able to identify the particular genes that cause postzygotic reproductive isolation. As a result, we were unable to address a
large set of fundamental questions about the factors that
cause reproductive isolation: are these factors ‘ordinary’
genes that have normal functions within species? If so, do
they typically belong to a particular functional class (e.g.
are they usually transcription factors)? Are these genes
rapidly diverging? And are they adaptively evolving?
Over the past several years, evolutionary geneticists,
capitalizing on the availability of genome sequences, have
finally succeeded in identifying and molecularly characterizing several genes that cause postzygotic reproductive
isolation. Here, we briefly review this recent work. As will
become clear, we consider that this research represents an
important new phase in the study of speciation, one that
unifies studies of the origin of species with studies of
molecular evolution.
Hybrid sterility
Several lines of evidence show that hybrid sterility
evolves faster than hybrid inviability. First, comparative
studies in various taxa have shown that sterility of hybrids
appears between diverging species earlier than does
inviability of hybrids [3,5,6,11]. Second, genetic studies
in Drosophila have shown that particular species pairs are
separated by more hybrid male sterility (HMS) genes
than either hybrid female sterility genes or hybrid inviability genes [12–15]. This excess of HMS genes might
arise as a byproduct of sexual selection [16], genetic
conflicts [14,17–21], or an intrinsic developmental sensitivity of spermatogenesis in hybrids [16]. The idea that
the genes causing HMS simply diverge faster between
species than do other types of genes is supported by the
more rapid evolution of both the DNA sequences and
expression levels of sex-related genes (or genes with sexbiased expression) relative to other genes [22–28]. There
would seem to be good reason, then, to expect a signature
of positive selection at the genes that cause hybrid
sterility.
The only HMS gene identified so far, Odysseus site homeobox (OdsH), causes sterility in male hybrids of Drosophila
simulans and Drosophila mauritiana. Using a classical
genetic approach, Coyne and Charlesworth [29] mapped
Current Opinion in Genetics & Development 2004, 14:675–679
676 Genomes and evolution
a segment of about 2 cM of the D. mauritiana X chromosome that causes HMS when introgressed into a D.
simulans genetic background. Using molecular markers,
Ting et al. [30] subsequently narrowed in on an 8.4kb
region including three exons. These three exons plus an
adjacent one encode OdsH, a protein that includes a
sequence of 60 amino acids characteristic of homeobox transcription factors. OdsH is a duplicate of unc-4,
which encodes a transcription factor expressed in the
embryo and in adult neural tissue. Although its function
within species remains unknown, OdsH has acquired
novel expression in testis. The homeobox motif of
OdsH suggests that it may cause HMS by misregulating
downstream target genes necessary for spermatogenesis.
Consistent with this, Michalak and Noor’s [31] recent
survey of gene expression has shown that backcross
hybrid males carrying a segment of D. mauritiana including OdsH show misexpression of at least five coordinately regulated loci, some of which function in
spermatogenesis.
Like many reproductive genes, OdsH has evolved rapidly.
The homeodomain alone has accumulated a remarkable
15 replacement substitutions (those that change the
amino acid encoded) in the roughly 0.25–1.00 million
years since D. simulans and D. mauritiana diverged from a
common ancestor [30]. The tenfold excess of replacement to silent substitutions in the lineage leading to
D. mauritiana strongly suggests that OdsH has evolved
by positive selection [30,32]. A burst of adaptive evolution of this magnitude is not seen in the lineage leading
to D. simulans, suggesting that selection pressures on
OdsH have differed in these two species.
When introgressed alone into D. simulans, the OdsH allele
from D. mauritiana causes a reduction in fertility of only
about 50%; to confer complete HMS, OdsH must be cointrogressed with unidentified but tightly linked factors
[30,33]. Findings from other genetic analyses also suggest
that HMS often has a polygenic basis [10,32]. There are,
however, exceptions. The tiny dot fourth chromosome of
D. simulans, for example, causes complete HMS when
introgressed into a Drosophila melanogaster genetic background [34,35]. Recent deletion mapping and complementation tests suggest (but do not prove) that the
D. simulans allele of a single gene causes this hybrid
sterility (JP Masly, unpublished).
Additional hybrid sterility loci will almost certainly be
identified in the near future. Tao et al. [19,36] have fine
mapped autosomal introgressions that cause both HMS
and hybrid meiotic drive in hybrids of D. mauritiana and
D. simulans. Similarly, Sawamura et al. [15,37] seem
poised to identify several hybrid sterility loci in hybrids
of D. melanogaster and D. simulans, in which five regions on
the second chromosome that cause HMS have been
localized by interspecific deletion mapping.
Current Opinion in Genetics & Development 2004, 14:675–679
Systems other than Drosophila may also soon yield hybrid
sterility loci. In Mus, for example, Dnahc8, an axonemal
dynein heavy chain expressed in testis, has been mapped
to the site of the Hybrid sterility 6 locus [38], which is
involved in HMS in hybrids of Mus domesticus and Mus
spretus. Thus, Dnahc8 represents a strong candidate hybrid
sterility locus.
Hybrid inviability
The evolutionary history of OdsH confirms what was
suspected from comparative and genetic data — namely,
that positive selection drives the rapid evolution of hybrid
sterility genes. The same comparative and genetic data
show, however, that hybrid inviability evolves more
slowly than does sterility in a range of taxa (see above).
It was therefore unclear whether positive selection would
also have a role in the evolution of hybrid inviability
genes; after all, hybrid incompatibilities could also evolve
through the slow accumulation of neutral or even slightly
deleterious substitutions. Although many evolutionary
details remain to be resolved for the first of the hybrid
inviability genes identified, two recently isolated genes
show clear signatures of adaptive evolution.
The first hybrid inviability gene to be identified was the
platyfish Xiphophorus melanoma receptor kinase 2 gene
(Xmrk2), which is located on the X chromosome [39–
41]. Xmrk2 encodes a novel receptor tyrosine kinase that
is overexpressed in some Xiphophorus hybrids, often causing lethal tumorigenesis. The evolutionary history of
Xmrk2 is complex. The gene originated more than 5
million years ago as a partial duplication and acquired a
novel 50 cis-regulatory region [42,43]. Although independently lost from several species, Xmrk2 persists as a
polymorphism in at least eight Xiphophorus species,
always in association with a tightly linked pigment pattern-encoding locus [43]. The persistence of this ancient
polymorphism through several speciation events and its
association with pigmentation patterning — a trait that is
known to influence mating success in these fish — may
indicate a history of balancing selection. Population
genetic analyses of the forces involved in the maintenance and evolution of Xmrk2 are needed.
The second hybrid inviability gene to be identified was
the Drosophila gene Hybrid male rescue (Hmr), which is
located on the X chromosome [44]. Crosses involving
D. melanogaster females and sibling species males (D.
simulans, Drosophila sechellia or D. mauritiana) yield dead
hybrid sons, whose development arrests at the larval–
pupal transition, and sterile hybrid daughters that die
when reared at high temperatures [45]. (Hmr also seems
to affect the fertility of hybrid daughters [46].) Hmr was
originally recovered in a screen for ‘rescue’ mutations in
D. melanogaster that can suppress hybrid inviability.
Nearly 20 years of effort have made Hmr the best characterized of all hybrid incompatibility genes. Genetic
www.sciencedirect.com
Speciation genes Orr, Masly and Presgraves 677
manipulations show that decreasing the Hmr dosage
suppresses the lethality of hybrid males and the temperature-dependent lethality of hybrid females, whereas
increasing Hmr dosage has the opposite effects [45,47].
Barbash et al. [44] found that Hmr encodes a protein
with two MADF DNA-binding domains similar to those
of myeloblastosis-related transcription factors. Recent
transgenic experiments confirm that the D. melanogaster
allele of Hmr has functionally diverged from those of
D. simulans and D. mauritiana [48].
The third hybrid inviability gene to be identified was the
Drosophila gene Nucleoporin-96 (Nup96), which is located
on the right arm of chromosome 3 [49]. The D. simulans
allele of Nup96 is incompatible with an unknown gene on
the X chromosome of D. melanogaster. Nup96 encodes 1 of
about 30 nuclear pore proteins (nucleoporins) that
together form nuclear pore complexes (NPCs), the
macromolecular structures that perforate nuclear membranes and conduct mRNA and protein trafficking
between the nucleus and cytoplasm. Nup96 is a one of
seven conserved interacting proteins of the Nup84 subcomplex [50,51]. From yeast to vertebrates, the Nup84
subcomplex is stably bound at the nuclear and cytoplasmic sides of the NPC, where it functions as a docking site
for dynamic nucleoporins that shuttle between the
nuclear interior and NPCs, delivering mRNA cargoes
for nuclear export [52].
It is worth considering the evolutionary histories of Hmr
and Nup96 together because they share several features.
First, both genes show clear evidence of adaptive protein
evolution: both have fixed more amino-acid-changing
substitutions between species than can be accommodated
by neutral models of evolution [48,49]. Second, both
genes have experienced adaptive evolution in the
D. melanogaster and D. simulans lineages. Last, these bouts
of adaptation seem to have been ancient because patterns
of polymorphism at both loci show no signs of a recent
selective sweep [48,49]. This does not, of course,
mean that adaptive evolution has necessarily ground to
a halt at these loci, only that no substitution has occurred
recently.
Future progress in hybrid inviability
Further progress in the molecular evolution and genetics
of hybrid inviability seems imminent. First, the success of
the Hmr work suggests that analogous analyses of other
hybrid rescue mutations should succeed. Second, Nup96
is only the first gene to emerge from a large deletionbased screen for autosomal D. simulans factors involved in
lethal incompatibilities with X-linked D. melanogaster
genes [53]. Nineteen other autosomal regions associated
with hybrid lethality have been mapped, and two of these
have been narrowed to single complementation groups
(DC Presgraves, unpublished). Last, conserved interactions among Nup84 subcomplex members [50,51] suggest
www.sciencedirect.com
natural candidates for the incompatible ‘partner’ molecules of Nup96. Once partners are identified for this and
other hybrid incompatibilities, evolutionary geneticists
can begin to analyze how coevolution among interacting
molecules within species ultimately gives rise to incompatible interactions between species.
Conclusions
Although it is too early to draw robust conclusions from
the small sample of ‘speciation genes’ isolated so far,
several facts seem reasonably clear. First, the factors that
cause postzygotic reproductive isolation are often ordinary genes that have normal functions within species.
Second, these genes are evolving rapidly. Last, and
perhaps most important, this rapid evolution is driven
by positive Darwinian selection. Recent molecular analyses of speciation genes thus support one of the central
tenets of the modern synthesis—namely, that reproductive isolation is an epiphenomenon of Darwinian selection within species.
We hasten to add, however, that the ecological basis of
this selection is often unclear. Indeed, we have no good
evidence that the relevant selection reflected adaptation
to the external environment at all, and it is entirely
possible that selection instead reflected adaptation to
the internal genetic ‘environment’, as posited by several
recent selfish gene theories of postzygotic isolation.
According to some of these theories, bouts of coevolution
between genes associated with meiotic drive and their
suppressors might drive the evolution of postzygotic
reproductive isolation [14,17–21].
One of the most important tasks facing speciation geneticists is therefore clear: we must connect the population
genetic signal of positive selection seen at speciation
genes to the biological basis of that selection. This goal
will require both the identification of more speciation
genes—a task facilitated by the increasing number of
genome sequences becoming available—and careful analyses of their normal functions within species.
Acknowledgements
This work was supported by a grant from the National Institutes
of Health (2R01GM51932) to HAO and by funding from the
Alexander von Humboldt Foundation to DCP.
References and recommended reading
Papers of particular interest, published within the annual period of
review, have been highlighted as:
of special interest
of outstanding interest
1.
Dobzhansky T: Genetics and the Origin of Species. New York:
Columbia University Press; 1937.
2.
Coyne JA, Orr HA: Patterns of speciation in Drosophila.
Evolution 1989, 43:362-381.
3.
Sasa MM, Chippindale PT, Johnson NA: Patterns of postzygotic
isolation in frogs. Evolution 1998, 52:1811-1820.
Current Opinion in Genetics & Development 2004, 14:675–679
678 Genomes and evolution
4.
Tilley SG, Verrell PA, Arnold SJ: Correspondence between
sexual isolation and allozyme differentiation — a test in the
salamander Desmognathus ochrophaeus. Proc Natl Acad Sci
USA 1990, 87:2715-2719.
26. Ranz JM, Namgyal K, Gibson G, Hartl DL: Anomalies in the
expression profile of interspecific hybrids of Drosophila
melanogaster and Drosophila simulans. Genome Res 2004,
14:373-379.
5.
Presgraves DC: Patterns of postzygotic isolation in
Lepidoptera. Evolution 2002, 56:1168-1183.
6.
Price TD, Bouvier MM: The evolution of F1 postzygotic
incompatibilities in birds. Evolution 2002, 56:2083-2089.
27. Nuzhdin SV, Wayne ML, Harmon KL, McIntyre LM: Common
pattern of evolution of gene expression level and protein
sequence in Drosophila. Mol Biol Evol 2004, 21:1308-1317.
7.
Mendelson TC: Sexual isolation evolves faster than hybrid
inviability in a diverse and sexually dimorphic genus of fish
(Percidae: Etheostoma). Evolution 2003, 57:317-327.
8.
Moyle LC, Olson MS, Tiffin P: Patterns of reproductive isolation
in four angiosperm genera. Evolution 2004, 58:195-1208.
9.
Orr HA: The population genetics of speciation: the evolution of
hybrid incompatibilities. Genetics 1995, 139:1805-1813.
10. Coyne JA, Orr HA: Speciation. Sunderland, MA: Sinauer
Associates; 2004.
A comprehensive review of the speciation literature, covering genetical,
evolutionary and ecological aspects.
11. Wu C-I, Johnson NA, Palopoli MF: Haldane’s rule and its legacy:
why are there so many sterile males? Trends Ecol Evol 1996,
11:281-284.
12. True JR, Weir BS, Laurie CC: A genome-wide survey of hybrid
incompatibility factors by the introgression of marked
segments of Drosophila mauritiana chromosomes into
Drosophila simulans. Genetics 1996, 142:819-837.
13. Hollocher H, Wu C-I: The genetics of reproductive isolation in
the Drosophila simulans clade: X vs autosomal effects and
male vs female effects. Genetics 1996, 143:1243-1255.
14. Tao Y, Hartl DL: Genetic dissection of hybrid incompatibilities
between Drosophila simulans and Drosophila mauritiana. III.
Heterogeneous accumulation of hybrid incompatibilities,
degree of dominance and implications for Haldane’s rule.
Evolution 2003, 57:2580-2598.
15. Sawamura K, Davis AW, Wu C-I: Genetic analysis of speciation
by means of introgression into Drosophila melanogaster.
Proc Natl Acad Sci USA 2000, 97:2652-2655.
16. Wu C-I, Davis AW: Evolution of postmating reproductive
isolation: the composite nature of Haldane’s rule and its
genetic bases. Am Nat 1993, 142:187-212.
17. Frank SH: Divergence of meiotic drive-suppressors as an
explanation for sex-biased hybrid sterility and inviability.
Evolution 1991, 45:262-267.
18. Hurst LD, Pomiankowski A: Causes of sex ratio bias may
account for unisexual sterility in hybrids: a new explanation of
Haldane’s rule and related phenomena. Genetics 1991,
128:841-858.
28. Zhang Z, Hambuch TM, Parsch J: Molecular evolution of
sex-biased genes in Drosophila. Mol Biol Evol 2004, in press.
29. Coyne JA, Charlesworth B: Location of an X-linked factor
causing male sterility in hybrids of Drosophila simulans and
D. mauritiana. Heredity 1986, 57:243-246.
30. Ting C-T, Tsaur S-C, Wu M-L, Wu C-I: A rapidly evolving
homeobox at the site of a hybrid sterility gene. Science 1998,
282:1501-1504.
31. Michalak P, Noor MAF: Association of misexpression with
sterility in hybrids of Drosophila simulans and D. mauritiana.
J Mol Evol 2004, in press.
32. Wu C-I, Ting C-T: Genes and speciation. Nat Rev Genet 2004,
5:114-122.
33. Perez DE, Wu C-I: Further characterization of the Odysseus
locus of hybrid sterility in Drosophila: one gene is not enough.
Genetics 1995, 140:201-206.
34. Muller HJ, Pontecorvo G: Recessive genes causing
interspecific sterility and other disharmonies between
Drosophila melanogaster and simulans. Genetics 1942, 27:157.
35. Orr HA: Mapping and characterization of a ‘‘speciation gene’’
in Drosophila. Genet Res 1992, 59:73-80.
36. Tao Y, Zeng Z-B, Li J, Hartl DL, Laurie CC: Genetic dissection of
hybrid incompatibilities between Drosophila simulans and
D. mauritiana. II. Mapping hybrid male sterile loci on the third
chromosome. Genetics 2003, 164:1399-1418.
The authors carry out a large and fine-scale introgression study of hybrid
male sterility factors on the third chromosome of D. mauritiana in a genetic
background that is largely D. simulans. This work represents the highest
resolution analysis of this classic hybridization so far performed.
37. Sawamura K, Roote J, Wu C-I, Yamamoto M-T: Genetic
complexity underlying hybrid male sterility in Drosophila.
Genetics 2004, 166:789-796.
This paper presents a fine-scale deficiency-mapping analysis of hybrid
male sterility factors located on a small region of chromosome 2 of
D. simulans introgressed onto an otherwise D. melanogaster genetic
background.
38. Fossella J, Samant SA, Silver LM, King SM, Vaughan KT,
Olds-Clarke P, Johnson KA, Mikami A, Vallee RB, Pilder SH:
An axonemal dynein at the Hybrid sterility 6 locus: implications
for t haplotype-specific male sterility and the evolution of
species barriers. Mamm Genome 2000, 11:8-15.
19. Tao Y, Hartl DL, Laurie CC: Sex-ratio distortion associated with
reproductive isolation in Drosophila. Proc Natl Acad Sci USA
2001, 98:13183-13188.
39. Wittbrodt J, Adam D, Malitschek B, Maueler W, Raulf F, Telling A,
Robertson SM, Schartl M: Novel putative receptor tyrosine
kinase encoded by the melanoma-inducing Tu locus in
Xiphophorus. Nature 1989, 341:415-421.
20. Henikoff S, Ahmad K, Malik HS: The centromere paradox: stable
inheritance with rapidly evolving DNA. Science 2001,
293:1098-1102.
40. Schartl A, Dimitrijevic N, Schartl M: Evolutionary origin and
molecular biology of the melanoma-inducing oncogene of
Xiphophorus. Pigment Cell Res 1994, 7:428-432.
21. Henikoff S, Malik HS: Selfish drivers. Science 2002,
417:227.
41. Malitschek B, Fornzler D, Schartl M: Melanoma formation in
Xiphophorus: a model system for the role of receptor tyrosine
kinases in tumorigenesis. BioEssays 1995, 17:1017-1023.
22. Swanson WJ, Vacquier V: Rapid evolution of reproductive
proteins. Nat Rev Genet 2002, 3:137-144.
23. Reiland J, Noor MAF: Little qualitative RNA misexpression in
sterile male F1 hybrids of Drosophila pseudoobscura and
D. persimilis. BMC Evol Biol 2002, 2:16.
24. Michalak P, Noor MAF: Genome-wide patterns of expression in
Drosophila pure species and hybrid males. Mol Biol Evol 2003,
20:1070-1076.
25. Ranz JM, Catillo-Davis CI, Meiklejohn CD, Hartl DL:
Sex-dependent gene expression and evolution of the
Drosophila transcriptome. Science 2003, 300:1742-1745.
Current Opinion in Genetics & Development 2004, 14:675–679
42. Adam D, Dimitijevic N, Schartl M: Tumor suppression in
Xiphophorus by an accidentally acquired promoter.
Science 1993, 259:816-819.
43. Weis S, Schartl M: The macromelanophore locus and the
melanoma oncogene Xmrk are separate genetic entities in the
genome of Xiphophorus. Genetics 1998, 149:1909-1920.
44. Barbash DA, Siino DF, Tarone AM, Roote J: A rapidly evolving
MYB-related protein causes species isolation in Drosophila.
Proc Natl Acad Sci USA 2003, 100:5302-5307.
The authors identify the Drosophila hybrid inviability gene, Hybrid male
rescue, and show that it has diverged very rapidly between species.
www.sciencedirect.com
Speciation genes Orr, Masly and Presgraves 679
45. Barbash DA, Roote J, Ashburner M: The Drosophila
melanogaster Hybrid male rescue gene causes inviability
in male and female species hybrids. Genetics 2000,
154:1747-1771.
46. Barbash DA, Ashburner M: A novel system of fertility rescue in
Drosophila hybrids reveals a link between hybrid lethality and
female sterility. Genetics 2003, 163:217-226.
47. Orr HA, Irving S: Genetic analysis of the Hybrid male rescue
locus of Drosophila. Genetics 2000, 155:225-231.
48. Barbash DA, Awadalla P, Tarone AM: Functional divergence
caused by ancient positive selection of a Drosophila hybrid
incompatibility locus. Public Library of Sciences 2004,
2:839-848.
49. Presgraves DC, Balagopalan L, Abmayr SM, Orr HA: Adaptive
evolution drives divergence of a hybrid inviability gene
between two species of Drosophila. Nature 2003, 423:715-719.
The Drosophila hybrid inviability gene Nup96 is identified and characterized. Nup96 is shown to have a history of adaptive evolution in both the
D. melanogaster and D. simulans lineages.
www.sciencedirect.com
50. Siniossoglou S, Lutzmann M, Santos-Rosa H, Leonard K,
Mueller S, Aebi U, Hurt E: Structure and assembly of the
Nup84p complex. J Cell Biol 2000, 149:41-54.
51. Belgareh N, Rabut G, Bai SW, van Overbeek M, Beaudouin J,
Daigle N, Zatsepina OV, Pasteau F, Labas V, Fromont-Racine M
et al.: An evolutionarily conserved NPC subcomplex, which
redistributes in part to kinetochores in mammalian cells.
J Cell Biol 2001, 154:1147-1160.
52. Griffis ER, Craige B, Dimaano C, Ullman KS, Powers MA: Distinct
functional domains within nucleoporins Nup153 and Nup98
mediate transcription-dependent mobility. Mol Biol Cell 2004,
15:1991-2002.
53. Presgraves DC: A fine-scale genetic analysis of hybrid
incompatibilities in Drosophila. Genetics 2003,
163:955-972.
This paper presents a large deficiency-based screen for autosomal genes
from D. simulans that are incompatible with X-linked genes from D.
melanogaster, causing hybrid inviability. The authors identify 20 regions
associated with hybrid lethality.
Current Opinion in Genetics & Development 2004, 14:675–679