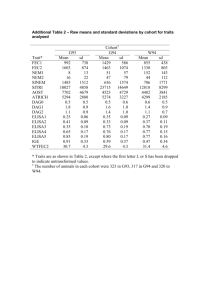
Descriptive Studies Types of Descriptive Studies

1.
2.
3.
1.
2.
3.
-
-
II
-
-
I.
II.
I.
Descriptive Studies
These are a type of observational studies where in the investigator observes the events occurring in the population without doing any interruption or manipulation. They aim at disease occurrence or exposure to risk factors with respect to
- Person distribution
- Place distribution
- Time distribution
They form the first step in identifying risk factors.
Provide clues for generating hypothesis.
In these studies there is no formal comparison groups.
Types of Descriptive Studies
Cross Sectional Studies.
Longitudinal Studies.
Cross Sectional Studies :
Are based on a single examination of a cross-section of population at one point in time.
Results can be projected on the whole population provided the sampling has been done randomly.
A series of cross sectional studies done at several points in time is known as serial survey design.
Cross sectional studies are relatively fast and inexpensive and form only design to give prevalence of disease.
4.
Longitudinal Studies:
Observations are repeated in the same population over a prolonged period of time by means of follow up examinations. Useful to study
Natural h/o disease and its future outcome.
For identifying risk factors of disease.
For finding out incidence rate.
Steps for Conducting Cross Sectional Studies
Define the objectives of the study.
Define the population from whom sample will be selected.
Define the disease / health problem to be studied.
It should be an operational definition. Diagnostic criteria should be laid down.
Decide the design for drawing the sample from the population – Random sample.
b. c. d. e.
5.
6.
7.
8.
9.
10.
11. a.
Make the list of variables to be studied.
Prepare the questionnaire.
Decide the sample size.
Collection of data – by using pre-tested questionnaire.
Measurement on study subjects to be made on single occasion.
Analysis – each variable to be analyzed separately and in relation to the relevant variable in pairs.
Formulation of hypothesis.
Advantages of Descriptive Studies
Provide data in terms of magnitude of the disease
Morbidity
Mortality
Provide clues to disease aetiology
Help to generate hypothesis, which can further be tested by analytical studies.
Provide data for planning, organizing and evaluating preventive and curative services.
Contribute to research in terms of disease occurrence by time, place and person.
We are all working together to one end, some with knowledge and design, and others without what they do.
-
Macrus Aurelius
COHORT STUDIES
♦
COHORT STUDIES aim at testing HYPOTHESIS ( which is obtained by either descriptive studies / Review of Literature.
♦
These studies give us a direct estimate of incidence rate, relative risk and attributable risk
(that is strength of association between a disease and a suspected causative factor).
♦
COHORT – Means a group of persons possessing a Characteristic in Common
E.g. – Birth Cohort / Marriage Cohort
♦
One should have a clearly stated HYPOTHESIS before designing a Cohort Study.
♦
Cohort studies proceed from CAUSE EFFECT
♦
Study is carried out on HEALTHY PEOPLE (Exposure has occurred, but the disease has not).
DESIGN OF A COHORT STUDY
POPULATION
PEOPLE WITHOUT DISEASE
DISEASE
EXPOSED
NO DISEASE
UNEXPOSED
DISEASE NO DISEASE
a) b) c) d) e)
Firstly choose a COHORT either from a general population (to study common exposures) or from a special group of people (to study rare exposures but they are HEALTHY
PEOPLE
Then divide them into two groups Exposed and Unexposed (Define what is exposure first)
Match both groups in respect to all the possible variables, which nay influence the frequency of the disease occurrence.
Define DISEASE (outcome) and its diagnostic criteria beforehand, clearly and objectively.
Then follow up both the groups similarly and look for the disease (outcome) at the end of the defined period of time (6 months – several years)
ANALYSIS
♦
Estimation of INCIDENCE RATE
♦
Estimation of RISK a) RELATIVE RISK b) ATTRIBUTABLE RISK
(First tabulate the data obtained in a simple two by two table as shown below)
COHORT
Exposed
Unexposed
YES
A
NO
B
DISEASE
TOTAL a + b
C D c + d
II
III
I
n = sample size = a + b + c + d.
(Now, apply the following simple formulas to calculate the parameters) a) Incidence rate
(Exposed)
a
= x 100
a + b b) Incidence rate
(Unexposed)
Relative Risk
Attributable Risk
=
=
c
= x 100
c + d a / a + b c / c + d a / a + b – c / c + d
a / a + b x 100
ADVANTAGES
♦
Gives direct estimation of incidence rate, relative risk and attributable risk.
♦
Several possible outcomes related to exposure can be studied simultaneously
♦
One can study the natural history of disease under question and also time
taken for development of such disease.
♦
Excellent model to study rare exposures
DISADVANTAGES
♦
May involve long term follow up
♦
Relatively expensive
♦
Loss to follow up
♦
Exposure status may change during the course of study
The faculties developed by doing RESEARCH are those most needed in DIAGNOSIS
- F.H. Adler
Discovery consists of seeing what everybody has seen, and thinking what nobody has thought.
-
Albert Szent Gyorgyi
CASE CONTROL STUDIES
♦
Is an analytical study, which is going to test the Hypothesis.
♦
It starts from a HEALTH CONDITION (DISEASE) and goes backwards through MEDICAL
RECORDS.
♦
Is done to find a cause in diseased persons where the disease is rare with long latent period.
RARE INCIDENCE < 5%
LONG LATENT PERIOD 10-15 YEARS
E.g.: Malignancy, HIV etc.
♦
This Study is:
Less Expensive
Less time consuming
Done on the basis of medical records
Identifies other potential risk factors of the disease
♦
The study should have GOOD INFRASTRUCTURE AND GOOD MEDICAL RECODRS maintained.
♦
This study includes : a) CASES: Who have the health problem (Disease). b) CONTROLS: Who do not have the health problem. c) A common exposure (cause) for both the cases and controls.
♦
The study determines :
That Exposure (cause) is responsible for the health condition (Disease) and is done by testing the hypothesis
♦
Eligibility of Cases:
-
-
-
Who have the health problem
All new cases diagnosed over a specified time
With well defined Diagnostic criteria
- Signs and symptoms
- Laboratory tests
- Diagnostic procedures should prove the diagnosis positively
YES / NO
♦
Eligibility of Controls: No health problem
Are matched with the cases
Selected randomly from population / hospitals / clinic / community etc.
♦
Always avoid over or under representation by a) Proper selection of cases b) Avoid incorrect information
It should be YES / NO but not MAY BE
Exposure past
DESIGN OF CASE CONTROL STUDY
CASE CONTROL STUDY
Definition of Comparison Groups in Control Studies
Case
Study Subjects
(Identified according to disease status or outcome)
Controls
(Comparison Groups) present
EXAMPLE
EXAMPLE A
Oral Contraceptives Cause Breast Cancer:
With Breast Cancer
(Cases)
From the same Hospital.
Oral Contraceptives
(Exposure)
Without Breast Cancer
(Controls)
Yes
Disease
Yes
(case) a
No
(control) c
Total a + c
ANALYSIS
Analysis: Case-Control Studies
Exposure
No b
Total a + b d c + d
b + d N = a + b + c + d
6.
7.
1.
2.
3.
4.
5.
1)
2)
3)
Odds Ratio (Cross – products ratio) = a d / b c
♦
Advantages
1)
2)
Useful for studying health problem that occur infrequently
Less time consuming & less expensive
3) Identify other potential risk factors
♦
Disadvantages
Data are collected from records or by recall after the disease has occurred
This study cannot be used to determine the other possible health effects of an exposure
Bias cannot be totally avoided
STEPS OF THE STUDY
Hypothesis
Rare Health Problem and Long latent Period
Accurate Medical Records
Define Diagnostic Criteria
Divide into 2 groups a) Cases b) Controls
Collection of Data
Analysis of Odds Ratio
With a Common Exposure