PROPERTIES of GASES
advertisement
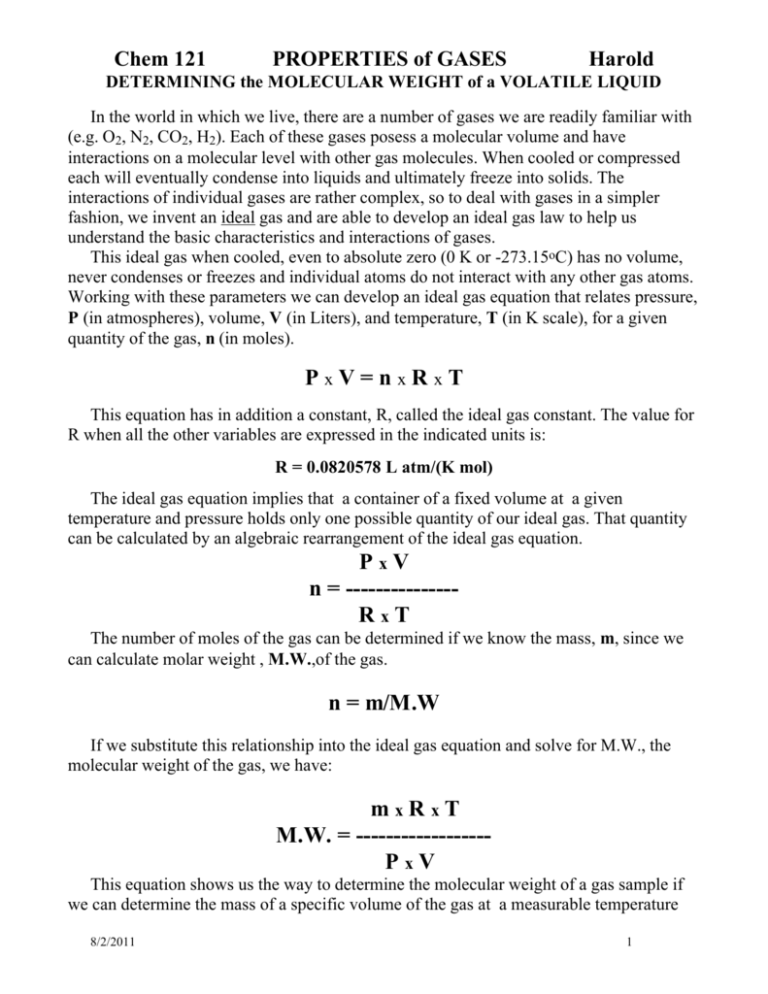
Chem 121 PROPERTIES of GASES Harold DETERMINING the MOLECULAR WEIGHT of a VOLATILE LIQUID In the world in which we live, there are a number of gases we are readily familiar with (e.g. O2, N2, CO2, H2). Each of these gases posess a molecular volume and have interactions on a molecular level with other gas molecules. When cooled or compressed each will eventually condense into liquids and ultimately freeze into solids. The interactions of individual gases are rather complex, so to deal with gases in a simpler fashion, we invent an ideal gas and are able to develop an ideal gas law to help us understand the basic characteristics and interactions of gases. This ideal gas when cooled, even to absolute zero (0 K or -273.15oC) has no volume, never condenses or freezes and individual atoms do not interact with any other gas atoms. Working with these parameters we can develop an ideal gas equation that relates pressure, P (in atmospheres), volume, V (in Liters), and temperature, T (in K scale), for a given quantity of the gas, n (in moles). PxV=nxRxT This equation has in addition a constant, R, called the ideal gas constant. The value for R when all the other variables are expressed in the indicated units is: R = 0.0820578 L atm/(K mol) The ideal gas equation implies that a container of a fixed volume at a given temperature and pressure holds only one possible quantity of our ideal gas. That quantity can be calculated by an algebraic rearrangement of the ideal gas equation. PxV n = --------------RxT The number of moles of the gas can be determined if we know the mass, m, since we can calculate molar weight , M.W.,of the gas. n = m/M.W If we substitute this relationship into the ideal gas equation and solve for M.W., the molecular weight of the gas, we have: mxRxT M.W. = -----------------PxV This equation shows us the way to determine the molecular weight of a gas sample if we can determine the mass of a specific volume of the gas at a measurable temperature 8/2/2011 1 Chem 121 PROPERTIES of GASES Harold DETERMINING the MOLECULAR WEIGHT of a VOLATILE LIQUID and pressure. Most real gases at low pressures (about 1 atm or less) and relatively high temperatures (300 K or more) behave very much like ideal gases. Thus, under these conditions of high temperature and low pressure, the ideal gas law will work well for real gases. In this experiment a small quantity of a volatile liquid will be vaporized in a tared flask of measurable volume. Since the boiling point of the gas chosen is below that of boiling water, when the flask is submerged in a boiling water bath, the liquid will vaporize completely. In the process of vaporizing the gas will drive out atmospheric gases trapped in the flask. The flask will then be completely filled with the vapor of the volatile liquid. All the volatile liquid will be vaporized and the temperature of the gas in the flask will be allowed to equilibrate with that of the boiling water and with the atmospheric pressure. Thus the temperature can be measured by measuring the water's temperature. The pressure can be detemined by a barometer. The flask is allowed to cool. The vapor of the volatile liquid will now condense to liquid droplets and the space inside the flask will be filled again with atmospheric gases. Since the flask was tared originally with atmospheric gases, reweighing the flask will give us the weight of the condensed vapor. We now have sufficient data to calculate the molar weight of the volatile gas. Do the following two examples as the pre-lab exercises. PRE-LAB EXERCISES 1. A sample of an unknown liquid is vaporized in an Erlenmeyer flask of volume 250. mL. At 100.oC the vapor has a pressure of 0.974 atm. The condensed vapor weighs 0.685 g. Determine the molecular weight of the unknown liquid and from the Table listed further on in the lab pages determine the identity of the gas. 2. Another sample of an unknown liquid was similarly vaporized in a boiling water bath as before. The pressure and volume were the same as in the first example (the same flask was used and outside air pressure had not changed). The mass of the condensed liquid was measured to be 0.452 g. Again determine molar weight of the gas and it's identity. PURPOSE 8/2/2011 2 Chem 121 PROPERTIES of GASES Harold DETERMINING the MOLECULAR WEIGHT of a VOLATILE LIQUID 1. To experimentally determine the mass of the vapor of a volatile liquid in a measurable volume. 2. To calculate the molar weight of the liquid using the ideal gas equation. 3. To determine the name of the specific volatile liquid you have been given. The table below should be used to determine which gas/volatile liquid you have been working with. All the compounds listed below have a B.P. below that of water and could be used in this particular experiment. __________________________________________________________________ LIQUID FORMULA B.P. MOLAR WEIGHT (grams) o _____________________________ C_____________________________________ Methanol CH3OH 64.7 32.0 Ethanol C2H5OH 78.5 46.1 Acetone CHO(CH3)2 56.5 58.1 Propanol C3H7OH 82.3 60.1 Pentane C5H12 36.2 72.2 Hexane C6H14 69.0 86.2 __________________________________________________________________ PROCEDURES 1. Take a 600 mL beaker and add ~250 mL water and a few boiling chips (save these when done with lab). Heat the water on a hot plate (no bunsen burners, as most of the unknowns are quite flammable). Adjust the hot plate so that the water boils but does not splash, i.e., not too vigorously(so NOT high). 2. Obtain the unknown sample from the cart at back of lab. Record the unknown's number on the report sheet (1). 3.As one unit weigh the following and record the total weight (2), to the nearest 0.0001g: a clean, dry 125 mL Erlenmeyer flask, a square of aluminum foil and a rubber band (keep these together). 4. Pour approximately 3 mL of the unknown liquid into the flask. Cover the mouth of the flask with the square of aluminum foil and crimp the edges tightly around the neck of the flask. Seal the foil with the first rubber band wrapped around the neck tight. 5. Take a larger piece of aluminum foil and secure a second covering over the first using a second rubber band to hold it in place. This second piece is designed to protect the first from water which would alter the flask mass so it MUST completely cover the first foil cover. 6. Carefully punch one very small hole through both of the covers using a pin or needle. (see figure 1 below) 7. Immerse the flask containing the volatile liquid in the boiling water. Ensure that the volatile liquid is below the surface of the boiling water. (see figure 2 below) 8/2/2011 3 Chem 121 PROPERTIES of GASES Harold DETERMINING the MOLECULAR WEIGHT of a VOLATILE LIQUID Fig. 1 Fig. 2 8. Observe the unknown liquid. There is more than enough liquid present to fill the flask with the gas as it evaporates. The flask began with primarily atmospheric gases in it. As the volatile liquid evaporates these gases will be forced out the pin hole and the unknown will fill the space.. When all the liquid appears to be gone continue boiling for ~3 more minutes. 9. Measure and record the temperature of the boiling water (5). Record the temperature of the vapor in the flask (6) Q – how must the two be related? 10. Using tongs, carefully remove the flask from the water and set it on the laboratory bench. 11. While the flask is cooling to room temperature, record the barometric pressure in the lab.(7) 12. When the flask has cooled to room temperature, wipe the outside dry with a paper towel. Then carefiully remove the second foil cover and rubber band. Carefully blot dry the outside of the first cover, leaving it in place. Notice the inside of the flask, there should be droplets of liquid present. 13. Weigh the flask with the first cover intact. Record to the nearest 0.0001 g on the Report sheet (3) 14. Remove the foil cover and rubber band but do not discard. Rinse the flask and fill to the rim with water. Be sure no extra water is on the outside of the flask. Weigh the flask with water and the first foil cover with rubber band all together on the top-loader and record the weight to the nearest 0.01 g (8) 15. Determine the weight of the water (9). Determine the temperature of the water in the flask (10). Calculate the density of water at the recorded temperature using the equation below. Then, calculate the volume of the water in the flask.(11) density = -2.32x10-4T + 1.00287 where T is the recorded room temperature in C. 16. Calculate the molar weight of the unknown (12). Identify the unknown (13). 8/2/2011 4 Chem 121 PROPERTIES of GASES Harold DETERMINING the MOLECULAR WEIGHT of a VOLATILE LIQUID Name:_________________________ Partner:___________________________ DATA and CALCULATIONS 1. Unknown number _____________ 2. Weight of 125 mL Erlenmeyer flask, Al foil and rubber band. ____________g 3. Weight of cooled flask, foil, rubber band and condensed liquid ____________g 4. Weight of condensed liquid: #3 - #2 ____________g 5. Temperature of boiling water ____oC ____K 6. Temperature of vapor in flask ____oC ____K 7. Barometric pressure ______Torr _______atm 8. Weight of 125mL Erlenmeyer, foil, rubber band and H2O ____________g 9. Weight of water: #8 - #2 ____________g 10. Temperature of water (i.e. room temp) ______oC 10.a) Density of H2O at temperature in #10 _________g/mL 11. Volume of the flask: ___________mL #9/(#10a) 12. Molecular weight (#4 x 0.0820578 L atm/K mole x #6) of unknown M.W. = ---------------------------------------________g/mole (#7 x #11) Show work M.W. = 13. Identity of unknown volatile liquid Give your reasoning. __________________ POST-LAB QUESTIONS 8/2/2011 5 Chem 121 PROPERTIES of GASES Harold DETERMINING the MOLECULAR WEIGHT of a VOLATILE LIQUID 1. If 1.15g of a gas occupies a 500 mL flask at 30.oC and 750. mm Hg of pressure, what is the molecular weight of the gas? (be sure to use or convert to proper units) 2. What assumptions are made concerning the chemical nature of the gas trapped in the Erlenmeyer flask? (Hint – what Law is the entire calculation based on) 3. If we used a 125 mL Erlenmeyer flask why do we need to determine the volume of the flask? In otherwords why not simply use 125 mL as our volume? 4. Suppose that all the liquid was not vaporized in the flask before the flask was removed from the boiling water. How would this affect the calculated molecular weight, or ? Explain 5. Suppose that water remains on the outside of the flask when the flask containing the recondensed liquid is weighed. How would this affect the calculated molecular weight, or ? Explain 8/2/2011 6






