Adam Seabrease Experiment 6 EDTA Titration of the Hardness of
advertisement

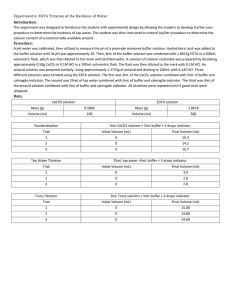
Adam Seabrease Experiment 6 EDTA Titration of the Hardness of Water Introduction: In this experiment, we tested the hardness of local tap water. We used a complexation titration using ethylenediaminetetraacetic acid (EDTA) as the titrant to determine the amount of Ca2+ in the water. EDTA was used because EDTA has 1:1 stoichiometric ratio with Ca2+. Procedure: A pH meter, NaOH, and HCl were used to create an ammonia buffer with pH of 10. 5 mL of buffer was added to 1 g EDTA and diluted in 250 mL distilled water. In 100 mL HCl, .5 g CaCO3 was dissolved. In an Erlenmeyer flask, 5 mL of buffer and CaCO3 solution and 5 drops of hydroxynaphthnol blue indicator to standardize the EDTA. The EDTA was standardized thrice. The standardized EDTA was used to titrate 100 mL of tap water, 5 mL ammonia buffer and 5 drops of hydroxynaphthnol blue indicator three times. Three titrations were done using EDTA as the titrant, and 10 mL portions of antacid solution with 5 mL ammonia buffer and 5 drops of hydroxynaphthnol blue indicator. The antacid solution was made by dissolving an Tums antacid tablet in 100 mL 0.1M HCl. Data: EDTA mass: 1.0007 g CaCO3 mass: 0.5007 g Buffer pH: 10.08 Tums antacid mass: 1.2665 g Supposed Ca2+ in antacid: .400 g Titration Standardization Tap Water Antacid mL Used in Titration 1 21.03 4.28 16.13 mL Used in Titration 2 21.12 4.01 16.61 mL Used in Titration 3 21.52 3.98 16.26 Calculations: Concentration of EDTA: Titration 1 0.0119M Titration 2 0.0118M Titration 3 0.0116M Average 0.0118±0.0001M Titration 2 4.7266x10-4 Titration 3 4.6913x10-4 Average 4.8209x10-4±1.9476x10-4 Titration 2 0.0078±0.0001 Titration 3 0.0077±0.0001 Sum 0.0231±0.0002 Concentration Ca2+ in tap water: Titration 1 5.0449x10-4 Ppm of Ca2+ in tap water: Mass of Ca2+ in Tums: Titration 1 0.0076±0.0001 Weight Percent of Ca2+ in Antacid: Conclusion: We found there is very little Ca2+ in the local York tap water. We found that there is only 19.32 ppm. Also, we found there is not as much Ca2+ in a Tums antacid as the label says. This may be due to the loss of antacid in the mortar and pestle when the tablet was crushed.