North America Equity Research
13 January 2015
Initiation
Overweight
Juno Therapeutics
JUNO, JUNO US
Price: $61.51
A Lot of CARs in the Garage…Initiating at OW
Price Target: $66.00
We are initiating coverage of JUNO with an OW rating based on the potential of its
CAR-T platform, which we believe has broad and disruptive potential across oncology
and is at the forefront of the innovation cycle in biotech. The technology genetically
engineers a patient’s immune cells into “homing devices” that seek out and destroy
tumor cells. Enthusiasm is understandably high given unprecedented response rates in
hard-to-treat cancers, and this has been validated to a certain extent by the lineup of
major players with skin in the game (NVS, CELG, AMGN, PFE, JNJ). JUNO, in our
view, is particularly well positioned given its relationships with multiple leading
medical institutions, thus providing pipeline flexibility/optionality. Bottom line, the
CAR-T space is in its early stages, and while we do not think it’s necessary to pick a
winner just yet, we think JUNO may have a competitive advantage (plus wholly
owned products) that allows it to pull into the lead over time. Shares don’t come
cheap, but further data in 2015 as well as progress towards new targets/indications
should continue to make JUNO a name investors likely will want to own in 2015.
Biotechnology
Cory Kasimov
AC
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
Bloomberg JPMA KASIMOV <GO>
Whitney G Ijem
(1-212) 622-4668
whitney.g.ijem@jpmorgan.com
Brittany Terner
(1-212) 622-8527
brittany.terner@jpmorgan.com
J.P. Morgan Securities LLC
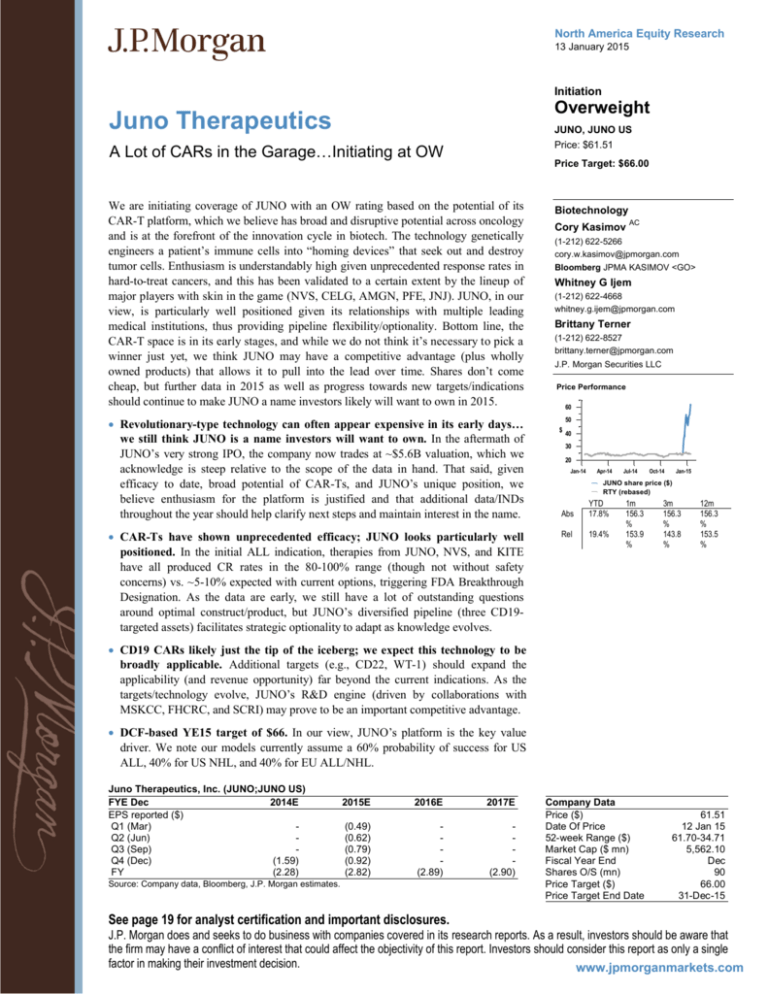
Price Performance
60
Revolutionary-type technology can often appear expensive in its early days…
we still think JUNO is a name investors will want to own. In the aftermath of
JUNO’s very strong IPO, the company now trades at ~$5.6B valuation, which we
acknowledge is steep relative to the scope of the data in hand. That said, given
efficacy to date, broad potential of CAR-Ts, and JUNO’s unique position, we
believe enthusiasm for the platform is justified and that additional data/INDs
throughout the year should help clarify next steps and maintain interest in the name.
50
$ 40
Abs
YTD
17.8%
CAR-Ts have shown unprecedented efficacy; JUNO looks particularly well
positioned. In the initial ALL indication, therapies from JUNO, NVS, and KITE
have all produced CR rates in the 80-100% range (though not without safety
concerns) vs. ~5-10% expected with current options, triggering FDA Breakthrough
Designation. As the data are early, we still have a lot of outstanding questions
around optimal construct/product, but JUNO’s diversified pipeline (three CD19targeted assets) facilitates strategic optionality to adapt as knowledge evolves.
Rel
19.4%
30
20
Jan-14
Apr-14
Jul-14
Oct-14
Jan-15
JUNO share price ($)
RTY (rebased)
1m
156.3
%
153.9
%
3m
156.3
%
143.8
%
12m
156.3
%
153.5
%
CD19 CARs likely just the tip of the iceberg; we expect this technology to be
broadly applicable. Additional targets (e.g., CD22, WT-1) should expand the
applicability (and revenue opportunity) far beyond the current indications. As the
targets/technology evolve, JUNO’s R&D engine (driven by collaborations with
MSKCC, FHCRC, and SCRI) may prove to be an important competitive advantage.
DCF-based YE15 target of $66. In our view, JUNO’s platform is the key value
driver. We note our models currently assume a 60% probability of success for US
ALL, 40% for US NHL, and 40% for EU ALL/NHL.
Juno Therapeutics, Inc. (JUNO;JUNO US)
FYE Dec
2014E
EPS reported ($)
Q1 (Mar)
Q2 (Jun)
Q3 (Sep)
Q4 (Dec)
(1.59)
FY
(2.28)
2015E
2016E
2017E
(0.49)
(0.62)
(0.79)
(0.92)
(2.82)
(2.89)
(2.90)
Source: Company data, Bloomberg, J.P. Morgan estimates.
Company Data
Price ($)
Date Of Price
52-week Range ($)
Market Cap ($ mn)
Fiscal Year End
Shares O/S (mn)
Price Target ($)
Price Target End Date
61.51
12 Jan 15
61.70-34.71
5,562.10
Dec
90
66.00
31-Dec-15
See page 19 for analyst certification and important disclosures.
J.P. Morgan does and seeks to do business with companies covered in its research reports. As a result, investors should be aware that
the firm may have a conflict of interest that could affect the objectivity of this report. Investors should consider this report as only a single
factor in making their investment decision.
www.jpmorganmarkets.com
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
Table of Contents
Investment Thesis ....................................................................3
Risks to Rating and Price Target ............................................5
Company Description ..............................................................6
Upcoming Events .....................................................................6
Pipeline......................................................................................7
Financial Outlook .....................................................................9
Valuation .................................................................................10
Management ...........................................................................12
Models .....................................................................................15
2
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
Investment Thesis
Juno Therapeutics
(JUNO)
Overweight
T-Cells may represent the future of cancer research
Immuno-oncology has been a very hot topic in cancer drug development for a few
years now, and its evolution continues. CAR-T technology is a type of
immunotherapy that combines the specificity of a monoclonal antibody with the
potent cell-killing activity of the immune system’s T cells. A CAR-T product
describes a cell-based therapy that comprises a patient’s own T cells that have been
genetically modified ex vivo (outside the body) to express chimeric antigen receptors
(artificial receptors that bind certain antigens with downstream effects that
affect/enhance the T cells’ behavior). When the cell product is infused into the body,
the CARs recognize and bind to specific markers expressed by target cells, and that
binding activates the T cells’ cytotoxic effects (which are enhanced via costimulatory domains of the CAR). The first wave of CAR-T products in the clinic
target CD19, a molecule expressed ubiquitously on B cells, though not on
hematopoietic cells. As CD19 expression is retained in many B-cell lymphomas and
leukemias, it represents an attractive target in the treatment of these diseases.
Unprecedented response rates with initial CD19 CAR-Ts are generating
significant enthusiasm among doctors, the industry, the FDA…and investors
The three highest-profile CAR-T programs are JUNO’s JCAR015 (MSKCC), NVS’s
CTL019 (UPENN) and KITE’s KTE-C19 (NCI). Each of these initial products target
CD19 and have shown impressive response rates in a variety of indications. In ALL
(acute lymphoblastic leukemia), CR rates in the 80-100% range have been observed
in highly refractory adult and pediatric patients (in whom CR rates of ~5-10% would
be expected with existing therapies). The FDA is also enthusiastic about the potential
of CAR-T therapies, having granted both NVS’s CTL019 and JUNO’s JCAR015
Breakthrough Therapy Designation (BTD) in 2014.
From “Model-Ts” to “Lamborghinis”…we expect significant evolution in both
the science and manufacturing of CAR-Ts in the coming years
We expect Juno, with its multitude of collaborations, to be at the forefront of this
revolution. As all of these products have looked impressive to date, we think it’s too
early (and likely not necessary) to pick a winner, and note that there is still a lot to
learn about 1) the optimal CAR-T construct (CAR structure/co-stimulatory domain,
cell composition of the therapeutic product, manufacturing procedure, etc.) as well as
2) the actual administration of the product (repeat doses, pre-conditioning, etc.).
JUNO’s pipeline of CD19 CAR-T products and multiple R&D collaborations
provide strategic flexibility that is relatively unique within the CAR-T space
From a competitive standpoint, we believe that JUNO is particularly well positioned
given its relationships with Memorial Sloan Kettering (MSKCC; JCAR015), the Fred
Hutchinson Cancer Research Center (FHCRC; JCAR014), and the Seattle Cancer
Research Institute (SCRI; JCAR017). Importantly, each of the three CD19-targeted
candidates has differences that allow JUNO to adapt to new information in choosing
the best product (not simply a potentially good one). We suspect this optionality
could facilitate a higher level of scientific integrity, and we are already seeing that
play out as JUNO has de-prioritized JCAR014 in favor of JCAR017 as its profile
may be more favorable in NHL. Such flexibility may be even more important longerterm as the technology and targets evolve.
3
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
CD19 likely just the beginning...the real excitement stems from the potential of
CAR-T therapy in the broader oncology space
While CD19 CAR-Ts in ALL have provided initial evidence of the exceptional
efficacy potential of this approach, this is likely just the tip of the iceberg (as well it
should be given the amount of money that is pouring into the field). JUNO and other
CAR-T players are investigating the potential of CARs (and TCRs, another type of
highly specific T-cell therapy) to target markers other than CD19 and across a range
of cancers. JUNO recently in-licensed a CD22-targeted program, and while this is
still directed towards B-cell malignancies, it represents JUNO’s first non-CD19
program in the clinic (Phase 1 to start imminently). Other targets identified by JUNO
include L1CAM, MUC-16/IL-12, ROR-1, and WT-1, which are all over-expressed in
certain cancer types and all of which are expected to be in Phase 1 in 2015.
We assume JCAR015 and JCAR017 are JUNO’s first commercial products and
model peak US sales of ~$150M in ALL and ~$1B in NHL
It is estimated that ~6,000 patients are diagnosed with ALL each year in the US,
~80% of which are of B-cell origin. We estimate roughly two-thirds of those patients
could ultimately be considered relapsed/refractory (across all lines of therapy), with
about half of patients ultimately being relapsed/refractory 2L+ (driven mostly by
adult patients). We assume an overall penetration of CAR-T into 2L+ rrALL of 90%
at peak (if it works, we think it will eventually overtake SCTs) and assume a price of
$250K. In NHL, we assume there are ~40K cases of aggressive disease diagnosed
each year in the US, with roughly one-third of those patients becoming relapsed/
refractory post 2L and eligible for treatment. We assign a slightly lower overall
CAR-T peak penetration of 75% in rrNHL. In both, we currently estimate JUNO gets
33% of the total CAR-T share (pending further data clarity).
Balance sheet: JUNO appears well positioned financially
JUNO ended 3Q14 with ~$238M in cash and subsequently raised ~$246M in an
initial public offering of common stock in December (J.P. Morgan acted as a co-lead
book-running manager). We estimate that JUNO will end 2014 with ~$430M in cash
and believe the company has sufficient capital through at least 2016.
Initiate at Overweight: JUNO well positioned within the nascent CAR-T space
with its collaborations and diversified platform of wholly owned products
We are initiating coverage of JUNO with an Overweight rating and year-end 2015
price target of $66. We believe in the long-term potential of CAR-Ts in general, and
see JUNO as uniquely well positioned given its relationships with multiple
institutions. Our target is based on a DCF analysis that projects FCF through 2022,
assumes a five-year intermediate growth rate of 15%, a terminal rate of 2.5%, and
utilizes a discount rate of 13%.
4
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
Risks to Rating and Price Target
Juno is susceptible to the standard risks that apply to the entire biotech industry,
including development, regulatory, commercial, manufacturing, financing, and IP
pitfalls. Risks more specific to Juno are outlined below:
Competitive risk
As highlighted above, there are an increasing number of players involved in the
CAR-T arena. It is possible that competitive programs may prove to be superior to
JUNO’s. On the other hand, it is possible that any safety issues/other problems with
other CAR-T programs may affect perception of JUNO’s programs. It is also
possible that resources available to competitive programs (driven by large Pharma
partners) could help accelerate development or otherwise benefit competitors.
Clinical risk
JCAR015, JCAR014 and JCAR017 have been used in a limited number of patients,
and there are still a lot of unanswered questions around the optimal CAR-T product.
It is possible that additional patients enrolled in JUNO’s trials may respond
differently to the various products, and ongoing trials may not meet the safety and
efficacy thresholds for further development. JUNO intends to initiate several
additional Phase 1 trials with non-CD19-targeted CARs, and it is possible that
efficacy in these new indications may not be as robust, and/or that previously
unknown safety issues could emerge with the new CARs.
Regulatory risk
As there are no currently approved CAR-T-based therapies, the hurdles for
regulatory approval may be higher/different than currently expected. Given CAR-T
products are living cells that respond to in vivo stimuli and grow, there may be
additional regulatory hurdles pre- or post-approval, and regulatory agencies may
want to see additional or longer-term data before approving one of JUNO’s therapies.
If approved, it is possible that the product labels may not be as anticipated,
potentially limiting use. Further, regulatory agencies could remove JCAR015,
JCAR017, or any other potentially approved product from the market if they show
additional/more severe AEs in a real-world setting.
Commercial risk
CAR-Ts are not simple therapies. They are more logistically complex than most
traditional anti-cancer therapeutics, including small molecules and traditional
monoclonal antibodies. Moreover, the striking potency of these products seems to
come at a price (at least in these early days) with potentially lethal cytokine release
syndrome (CRS) the most noteworthy complication. If approved, the complexity and
toxicity of CAR-Ts may relegate them to more niche opportunities than we currently
anticipate.
5
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
Company Description
Juno Therapeutics is a Seattle-based biopharmaceutical company focused on
developing therapies that re-engage the body’s immune system to fight cancer. The
company is developing cell-based immunotherapies based on its chimeric antigen
receptor (CAR) and T-cell receptor (TCR) technologies. JUNO’s technology
platform revolves around the ability to genetically modify T cells to recognize and
kill cancer cells. The company’s lead clinical programs, JCAR015, JCAR017, and
JCAR014, use CAR technology to target CD19, a protein expressed on the surface of
B cell leukemias and lymphomas. JCAR015 is currently in a Phase 1 trial in rrALL,
and JUNO intends to initiate a Phase 2 trial in 2015 that could support accelerated
approval in the US. The company intends to initiate a Phase 1/2 trial for JCAR017 in
rrNHL in 2015, as well as initiate several Phase 1 trials for new, non-CD19-targeted
products during the year.
Upcoming Events
Figure 1: JUNO News Flow Highlights
Anticipated Newsflow Highlights
Program
JCAR015
Event
Initiate pivotal Phase 2 trial in Adult ALL
Updated Data from Phase 1 trial in Adult rrALL
Expected
mid-2015
mid-2015
Significance
Low
High
JCAR017
Initiate Phase 1/2 trial in rrNHL
Updated Data from Phase 1/2 trial in pediatric rrALL
2015
mid-2015
Low
High
JCAR014
Updated Data from Phase 1/2 trial in B Cell malignancies
mid-2015
High
Source: Company reports and J.P. Morgan estimates.
For Juno in 2015, the key catalysts will be presentation of updated data from the
ongoing Phase 1 trial of JCAR015 in adult ALL, as well data updates from the Phase
1/2 trials of JCAR017 in pediatric ALL and JCAR014 in B-cell malignancies, all of
which we anticipate midyear at ASCO. We also expect additional data updates for
each program in December at ASH. During the year, JUNO also expects to initiate a
pivotal Phase 2 trial for JCAR015 in rrALL, as well as a Phase 1/2 trial of JCAR017
in adult rrNHL. After JUNO recently in-licensed a CD22 CAR from Opus Bio/NCI,
we anticipate initial data from the ongoing trial in rrNHL and rrALL in late 2015/
early 2016 (potentially at ASH). The company also expects to initiate Phase 1 trials
for at least three additional CAR product candidates including products targeting
L1CAM, MUC-16/IL-12, and ROR-1. JUNO intends to initiate a Phase 1 trial with
its WT-1-targeted TCR product in 2015.
6
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
Pipeline
Figure 2: JUNO Pipeline
Product Pipeline Highlights
Program
P/C
Ph 1
Ph 2
Ph 3
FDA
Mkt.
Partner
Comments
JCAR015
Adult ALL and Adult NHL
MSKCC
JCAR017
Pediatric ALL and Adult NHL
SCRI
JCAR014
Adult B Cell Malignancies
FHCRC
CD22 CAR
rrALL and rrNHL
NCI/Opus Bio
WT-1 TCR
AML, NSCLC
CD19 "Armored" CARs
CLL
CD19 Fully Human scFV CAR
Adult NHL
L1CAM CAR
Neuroblastoma
MUC16 & IL-12 "Armored" CAR
Ovarian Cancer
ROR-1 CAR
CLL, Solid Tumors
Source: Company reports.
Juno’s lead product candidates are CD19-targeted CAR products currently in Phase 1
and Phase 1/2 development.
JCAR015 – JCAR015 was developed at MSKCC; it makes use of a CD28 costimulatory domain, and the cell product is CD3+ enriched PBMCs. The drug is
currently being evaluated in a Phase 1 trial in adult rrALL patients. Data
presented at ASH 2014 (11/14/2017 cut-off), showed treatment with JCAR015
resulted in an 89% CR rate in 27 evaluable patients, with 78% of patients
achieving CRm. As has been the case with virtually all CD19-targeted CARs in
ALL, severe cytokine release syndrome (CRS) was the most notable AE; in 28
patients evaluable for safety, 28% experienced severe CRS (0% in patients with
low disease burden, 33% in patients with high disease burden). There have been
two deaths in patients with severe CRS. Protocol changes allowed for the use of a
lower dose of cells in patients with high-burden disease, and the safety/efficacy
of the lower dose is currently being evaluated, as is the safety/efficacy of a
second scheduled dose of cells roughly three weeks following the initial dose.
JCAR017 – JCAR017 was developed at SCRI and utilizes a 4-1BB costimulatory domain. The cell product is made up of a defined cell composition of
CD4+ and CD8+ T cells. A Phase 1/2 trial is currently ongoing in pediatric ALL
patients and recently updated data (11/26/2014 cut-off) show that 85% of the 13
treated patients achieved CR. Ten of the 11 CR patients remained in CR as of the
data cut-off date. About 39% of patients in the trial experienced CRS, and ~31%
of patients experienced febrile neutropenia. Interestingly, patients treated with
JCAR017 have experienced the highest cell expansion and longest cell
persistence in the body relative to JUNO’s other products. As those two metrics
7
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
are thought to correlate with clinical benefit, the company plans to take JCAR017
forward into adult rrNHL, as well as continue development in pediatric rrALL.
JCAR014 – JCAR014 was developed at FHCRC, and makes use of the 4-1BB
co-stimulatory domain, with the final product being composed of a defined ratio
of CD8+ and CD4+ T cells. JCAR014 is currently being evaluated in a Phase 1/2
trial in patients with rel/ref B Cell malignancies. The most recently presented data
is as of 11/25/2014. In 13 patients treated with rrALL, 11 received product with a
1:1 ratio of CD8+ to CD4+ cells (due to manufacturing issues). In those patients
who received the proper cell product, the investigator-reported CR rate is 100%,
with an 82% CRm rate. In rrNHL, ten of 12 patients received a cell product with
the proper ratio, and in those ten patients the investigator-reported CR+PR rate
was 60%. Initial data from this trial indicates that duration of response in rrNHL
correlates with cell expansion and cell persistence. As noted above, JCAR017 has
had the highest expansion/longest persistence, which is why JUNO is moving it
forward into rrNHL (and deprioritizing JCAR014). The company intends to
continue to enroll patients in the ongoing Phase 1/2 trial to explore additional
treatment strategies, though at this point does not intend to move JCAR014
forward into pivotal trials.
8
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
Financial Outlook
Juno Therapeutics is a developmental-stage biotechnology company with upcoming
clinical catalysts (Phase 1 updates for various products anticipated in mid-2015) with
potential for approval and launch of the latest-stage product (JCAR015, expected to
start a pivotal Phase 2 trial in 2015) in 2017. Currently, we do not model profitability
until 2019. Juno has worldwide commercialization rights to all of its CARs; the
company intends to commercialize in the US on its own, and we expect clarity on the
ex-US strategy in 2015. Given the highly targeted physician audience for the initial
indications, we currently assume the company commercializes JCAR015 and
JCAR017 on its own in the EU.
OpEx Expectations: Below we briefly highlight our assumptions for Juno’s key
operating spend line items.
COGS. Given Juno’s products are cell-based therapies, we expect biologic-like
COGS in the 15-30% range, though the company has indicated it is targeting the
lower end of that range. At peak, we model COGS of 25%, inclusive of ~10% in
royalties owed to the various academic collaborators.
R&D trends. We assume R&D will continue to ramp as Juno executes the
various early-stage trials, initiates a pivotal Phase 2 for JCAR015, and brings at
least four new products into the clinic. In the outer years, we assume the company
will continue to aggressively invest in R&D on additional products given its
technology platform and eventual commercial infrastructure.
SG&A trends. We anticipate Juno will begin to build commercial infrastructure
in 2017, ahead of the potential launch of JCAR015 which we assume will occur
during the year. Our model reflects an expected field force of ~20 reps to start.
Juno’s current cash position
should be sufficient through at
least 2016.
JUNO ended 3Q14 with ~$238 million in cash
JUNO ended 3Q14 with ~$238M in cash, and subsequently raised ~$246M in an
initial public offering of common stock in December (J.P. Morgan acted as a co-lead
book-running manager). We estimate that JUNO will end 2014 with ~$430M in cash,
and believe the company has sufficient capital through at least 2016.
Share count
We estimate Juno currently has ~93 million fully diluted shares outstanding,
including ~90 million common shares and ~3 million stock options post-offering.
Figure 3: JUNO Key Financial Metrics
Key Financial Metrics
In $ thousands
December financial year-end
Cash
Debt
2013A
2014E
2015E
2016E
2017E
2018E
2019E
36.1
-
459.0
-
135.0
-
122.9
-
153.6
-
37.9
-
(30.6)
(69.3)
150.0
(324.0)
-
(212.1)
200.0
(219.3)
250.0
(215.7)
100.0
(8.6)
-
Revenue
EPS
-
(3.37)
(2.82)
(2.89)
41.3
(2.90)
161.8
(1.93)
360.6
(0.31)
Average shares outstanding
Fully diluted shares outstanding
-
97.04
90.93
99.92
91.13
CFOp + CapEx (burn)
Expected financing
43.21
59.23
90.83
90.53
93.15
90.73
29.3
-
100.33
90.83
Source: Company reports and J.P. Morgan estimates.
9
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
Valuation
We are initiating coverage of
JUNO with an Overweight rating
and a December 2015 price
target of $66 per share.
Our year-end 2015 price target of $66 per share is based on a DCF analysis (100%).
Figure 4: JUNO Valuation Summary
Juno Valuation Summary
Discount rate
4Q15 Fully Diluted Shares (m)
13%
93.5
Main value drivers
US ALL
US NHL
Valuation methodology
DCF
P/E 2016
Real options scenario analysis
Risk adjusted NPV analysis
Total
Catal yst/liquidity discount
YE15 Price Target
Prob of approval
60%
40%
Peak WW sales est
(avg. scenario)
$
156
$ 1,250
Value / share
$
65.73
Weighting
100%
$
$
74.23
59.85
Avg peak yr
2021
2022
Adj. value/ share
$
65.73
0%
0%
$
$
$
$
65.73
0%
66
Source: J.P. Morgan estimates.
Discounted cash flow analysis (100% weighting)
We utilize a DCF to value JUNO. We believe this may prove to be more appropriate
longer-term than our NPV analysis given that it assigns some terminal value. With
this model, we project cash flows out to 2022 at which point we assign an
intermediate growth rate of 15% based on the potential for additional product
launches for five years followed by a terminal rate of 2.5% assuming continued
growth in revenues as additional products/indications come on line. Our DCF utilizes
a discount rate of 13%. We currently do not model for “IP expiry”-related revenue
erosion for this technology, and instead we think it’s eventually at greater risk of
some other potentially superior technology/approach coming along.
Figure 5: JUNO DCF Sensitivity Analysis
Termina l
Growth
Rate
$65.73
-2%
-1%
0.0%
0.5%
1.0%
1.5%
2.0%
2.5%
3.0%
3.5%
4.0%
8%
$102.60
$110.58
$120.56
$126.54
$133.38
$141.28
$150.49
$161.37
$174.43
$190.39
$210.35
9%
$88.40
$94.41
$101.76
$106.08
$110.94
$116.45
$122.75
$130.01
$138.49
$148.50
$160.52
10%
$76.77
$81.38
$86.92
$90.12
$93.69
$97.67
$102.14
$107.22
$113.02
$119.71
$127.52
Discount Rate
11%
12%
$67.11
$58.98
$70.70
$61.82
$74.96
$65.14
$77.39
$67.02
$80.06
$69.06
$83.01
$71.30
$86.30
$73.77
$89.96
$76.50
$94.09
$79.52
$98.77
$82.91
$104.12
$86.72
13%
$52.07
$54.34
$56.97
$58.44
$60.04
$61.77
$63.66
$65.73
$68.00
$70.52
$73.31
14%
$46.15
$47.99
$50.09
$51.26
$52.52
$53.87
$55.35
$56.95
$58.69
$60.60
$62.71
15%
$41.03
$42.53
$44.23
$45.17
$46.18
$47.26
$48.42
$49.68
$51.04
$52.51
$54.13
Source: J.P. Morgan estimates.
What’s the value of a novel technology platform with potential broad applicability?
In our view, this is currently the key question when considering the investment
merits of JUNO. At this stage in its maturation, we believe most of the value inherent
in JUNO is driven by its novel technology platform as opposed to any individual
product candidate. Although this value is admittedly subjective and difficult to
estimate (and is primarily based upon one’s view of comparable companies), we
believe the broad applicability and game-changing potential of Juno’s technology
needs to be taken into account. Not only has the platform been validated through the
10
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
generation of very exciting early-stage data and collaborations with multiple worldleading cancer research institutions, but we also believe the number of large
pharma/biotech companies entering the space (e.g., NVS, CELG, PFE, JNJ, and
AMGN) lends further credibility to this groundbreaking approach.
11
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
Management
Below we highlight key executives at Juno.
Hans Bishop, President and CEO
Hans Bishop is a co-founder of Juno and has served as CEO and a member of the
Board since September 2013. Mr. Bishop has also served as a member of the Board
of Avanir Pharmaceuticals (acquired by Otsuka Pharmaceutical) since May 2012.
Mr. Bishop previously served as Chairman of Genesis Biopharma from January 2012
until November 2012. From February 2012 until October 2012, Mr. Bishop was the
COO of PhotoThera (owned by Warburg Pincus), and he continued working with
Warburg Pincus as an Executive in Residence until October 2013. Mr. Bishop
previously served as EVP and COO at Dendreon from January 2010 to September
2011. Mr. Bishop has also served as the President of the Specialty Medicine Business
at Bayer Healthcare Pharmaceuticals from December 2006 to January 2010.
Mr. Bishop worked at Chiron Corp as SVP of Global Commercial Operations until
its sale to Novartis. Mr. Bishop received a B.Sc. in Chemistry from Brunel
University.
Steve Harr, M.D., CFO and Head of Corporate Development
Steve Harr is CFO and Head of Corporate Development and joined Juno in April
2014. Dr. Harr was Managing Director and Head of Biotechnology Investment
Banking at Morgan Stanley from May 2010 until he joined Juno. Prior to his
investment banking role at Morgan Stanley, Dr. Harr was Morgan Stanley’s lead
biotech research analyst and Co-Head of Global Healthcare Research. Dr. Harr
received a B.A. in Economics from College of the Holy Cross and an M.D. from The
Johns Hopkins University School of Medicine. Dr. Harr was a resident in internal
medicine at the University of California, San Francisco.
Mark Frohlich, M.D., EVP, Research & Development
Mark Frohlich is EVP, Research & Development, and joined Juno in February 2014.
Prior to joining Juno, Dr. Frohlich served as EVP of Research & Development and
CMO at Dendreon, where he served in various capacities beginning in August 2005.
Dr. Frohlich received a B.S. in Electrical Engineering and Economics from Yale
University and an M.D. from Harvard Medical School. Dr. Frohlich was a resident in
internal medicine and a fellow in oncology at the University of California, San
Francisco.
Mark Gilbert, M.D., CMO
Mark Gilbert is CMO and joined Juno in March 2014. Prior to joining Juno,
Dr. Gilbert served as an interim CMO or consultant in strategic drug development
and portfolio management in medical oncology for several US biotech and pharma
companies. Previously, Dr. Gilbert served as VP and Head Global Clinical
Development, Therapeutic Area Oncology, at Bayer Schering. Prior to Bayer
Schering, he held several executive positions with Berlex Pharmaceuticals and its
parent company Schering AG, mostly as VP and Head of Global Medical
Development Group, Oncology. Dr. Gilbert joined Berlex from Immunex.
Dr. Gilbert received a B.S. in Biochemistry from the University of Iowa and his
M.D. from the University of Iowa Medical School and trained in internal medicine,
12
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
infectious disease, and medical oncology at the University of California, San
Francisco, and the University of Washington, respectively.
Ken Mohler, Ph.D., CSO
Kendall Mohler is CSO and joined Juno in October 2013. Previously, Dr. Mohler
was CSO at ZetaRx Biotherapeutics. In addition, he was a co-founder of Trubion
Pharmaceuticals, and during his tenure served as SVP, R&D and CSO. Prior to
Trubion, Dr. Mohler served as VP of Biological Sciences at Immunex Corp, where
he was employed for 13 years. Dr. Mohler has published more than 35 manuscripts
and has four issued patents and six pending patent applications. Dr. Mohler received
a B.S. from the University of Kansas and a Ph.D. in Immunology from University of
Texas Health Science Center at Dallas, Southwestern Medical School.
Elizabeth Smith, SVP, Regulatory Strategy & Portfolio Management
Elizabeth Smith is SVP, Regulatory Strategy & Portfolio Management, and joined
Juno in November 2013. Ms. Smith has over 20 years of experience in the field of
regulatory affairs, quality, and manufacturing, with an emphasis on biologics and
advanced cellular therapies in oncology. Most recently, Ms. Smith served as the VP
of Regulatory Affairs at Dendreon and led the regulatory efforts resulting in FDA
licensure of Provenge. Prior to Dendreon, Ms. Smith held regulatory and
manufacturing positions at Genentech and Immunex. She holds B.A. in Biology from
Central Washington University.
Barney Cassidy, J.D., General Counsel and Secretary
Barney Cassidy is General Counsel and Secretary and joined Juno in January 2014.
Prior to joining Juno, Mr. Cassidy served in various roles at Tessera Technologies
from November 2008 to July 2013, including as its EVP, General Counsel, and
Secretary, and as President of Tessera Intellectual Property. He served in various
roles at Tumbleweed Communications from May 1999 to September 2008.
Mr. Cassidy practiced law at Wilson Sonsini Goodrich & Rosati from August 1992
to May 1999, and at Skadden, Arps, Slate, Meagher & Flom from September 1989 to
July 1992. Mr. Cassidy received a B.A. in Philosophy from the Jesuit House of
Studies, Loyola University, an M.A. in Philosophy from the University of Toronto,
and a J.D. from Harvard Law School.
Andy Walker, Ph.D., SVP, Manufacturing
Andrew Walker is SVP, Manufacturing, and joined Juno in April 2014. Prior to
joining Juno, Dr. Walker served in senior leadership roles at CMC Biologics. While
at CMC Biologics, Dr. Walker’s roles included serving as the Head of the GMP
Manufacturing Department. Dr. Walker also served as Head of the Process
Development organization where he oversaw all aspects of drug development. Over
the course of his career, Dr. Walker has worked on the development and
manufacturing of approximately 50 different biologic drug candidates. Andy earned
his Ph.D. in Chemical Engineering from the University of California, Berkeley, and a
B.S. in Chemical Engineering from the University of Washington.
Robin Andrulevich, VP, People
Robin Andrulevich is VP of People and joined Juno in October 2014.
Ms. Andrulevich has more than 20 years of experience in HR at high-growth
companies. Most recently, Ms. Andrulevich served as the Talent Director for the
early-stage technology venture capital firm, Madrona Venture Group.
13
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
Ms. Andrulevich also held several key, senior leadership human resources and talent
roles at Amazon and worked closely with its management team to significantly scale
the company’s growth. A graduate of the University of Connecticut, with a B.A. in
Communications Science, Ms. Andrulevich also attended Barnard College and
Columbia University.
14
North America Equity Research
13 January 2015
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
Models
Figure 6: JUNO Income Statement
Juno Therapeutics Income Statement
Cory Kasimov
cory.w.kasimov@jpmorgan.com
212.622.5266
Comments:
2013A
JCAR15 (Adult rrALL)
JCAR17 (peds rrALL and NHL)
Total Product Revnue
Other Rvenue
Total Revenues
-
COGS
R&D
SG&A
Litigation
GAAP Total Operating Expenses
Non-GAAP Total Operating Expenses
46.2
4.2
1.2
51.7
GAAP Operating Income
Non-GAAP Operating Income
Other income ( expenses)
GAAP pre-tax Income (loss)
Income Tax (benefit)
GAAP Net Income
GAAP Basic EPS
GAAP Diluted EPS
(51.7)
(0.1)
(51.8)
(51.8)
1Q14A
2Q14A
3Q14A
2014E
2015E
2016E
2017E
2018E
2019E
-
-
41.3
41.3
41.3
107.0
54.8
161.8
161.8
151.1
209.4
360.6
360.6
-
-
-
-
2.9
3.4
2.0
8.3
6.5
4.6
1.6
12.7
13.0
5.4
1.4
19.8
17.0
86.0
6.0
2.0
94.0
27.0
108.4
19.4
7.0
134.8
61.3
217.0
37.0
2.0
256.0
112.0
226.2
43.0
269.3
155.0
12.4
249.4
61.1
322.9
208.4
46.9
234.5
73.5
354.9
265.2
97.3
220.2
74.2
391.7
326.7
(8.3)
-
(12.7)
-
(19.8)
(17.0)
(94.0)
(27.0)
(94.0)
(94.0)
(1.59)
(1.59)
(134.8)
(61.3)
(10.7)
(145.5)
(145.5)
(3.37)
(3.37)
(256.0)
(112.0)
(256.0)
(256.0)
(2.82)
(2.82)
(269.3)
(155.0)
(269.3)
(269.3)
(2.89)
(2.89)
(281.6)
(167.1)
(281.6)
(281.6)
(2.90)
(2.90)
(193.1)
(103.4)
(193.1)
(193.1)
(1.93)
(1.93)
(31.2)
33.9
(31.2)
(31.2)
(0.31)
(0.31)
(27.0)
(27.0)
(0.46)
(0.46)
(72.0)
(72.0)
(1.67)
(1.67)
(112.0)
(112.0)
(1.23)
(1.23)
(155.0)
(155.0)
(1.66)
(1.66)
(167.1)
(167.1)
(1.72)
(1.72)
(103.4)
(103.4)
(1.03)
(1.03)
33.9
33.9
0.34
0.34
27.2
59.2
59.2
43.2
43.2
90.8
90.8
93.1
93.1
97.0
97.0
99.9
99.9
100.3
100.3
-
Non-GAAP pre tax income (loss)
Income Tax (benefit)
Non-GAAP Net Income
Non-GAAP Basic EPS
Non-GAAP Diluted EPS
Basic Shares Outstanding
Diluted Shares Oustanding
Margin Analysis:
Gross margin
Operating margin
Net margin
Tax Rate
Cost Analysis:
COGS as % of tot. prod. sales
R&D as % of tot. revenue
SG&A as % of tot. revenue
Year-over-year growth:
Total revenue
R&D Expense
SG&A Expense
Total operating expenses
Operating income
Net income
EPS
Basic Shares
Diluted Shares
4Q14E
NM
NM
NM
0.0%
NM
NM
NM
0%
NM
NM
NM
0%
NM
NM
NM
0%
NM
NM
NM
0%
NM
NM
NM
0.0%
NM
NM
NM
0.0%
NM
NM
NM
0.0%
70%
NM
NM
0.0%
71%
NM
NM
0.0%
73%
NM
NM
0.0%
NM
NM
NM
NM
NM
NM
NM
NM
NM
NM
NM
NM
NM
NM
NM
0.00%
NM
NM
0%
NM
NM
0%
NM
NM
30%
604.35%
147.94%
29%
144.89%
45.44%
27%
61.07%
20.57%
NM
#REF!
#REF!
#REF!
NM
NM
NM
NM
NM
NM
#REF!
#REF!
#REF!
NM
NM
NM
NM
NM
NM
#REF!
#REF!
#REF!
NM
NM
NM
NM
NM
NM
#REF!
#REF!
#REF!
NM
NM
NM
#REF!
NM
NM
#REF!
#REF!
#REF!
NM
NM
NM
#REF!
#REF!
NM
134.51%
357.36%
160.88%
160.88%
NM
#DIV/0!
#DIV/0!
#DIV/0!
NM
100.10%
90.89%
89.89%
89.89%
NM
-16.31%
110.19%
#DIV/0!
NM
4.25%
16.30%
5.18%
5.18%
NM
2.56%
2.55%
0.88%
NM
10.27%
41.90%
19.92%
4.59%
NM
0.40%
4.18%
4.21%
292.07%
-6.00%
20.44%
9.93%
-31.43%
NM
-33.40%
2.97%
4.15%
122.80%
-6.09%
0.86%
10.36%
-83.86%
NM
-83.93%
0.42%
0.92%
<--- Assume core R&D spend
continues to increase;
outer year decline due to
lower success payment
liability
Source: Company reports and J.P. Morgan estimates.
15
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
Figure 7: JUNO Balance Sheet
Juno Therapeutics Balance Sheet ($ millions)
Cory W. Kas imov
cory.w.kasimov@jpmchase.com
212.622.5216
2013A
2014E
2015E
2016E
2017E
2018E
2019E
Assets
Cash and cash equivalents
Prepaid Expenses
Total Current Assets
PPE, Net
Fair value of covertible preferred stock
Other
Total Assets
36.0
0.2
36.1
0.0
3.8
0.1
40
459.0
0.2
459.2
0.0
459
135.0
0.2
135.2
15.0
-
122.9
0.2
123.1
30.0
-
153.6
0.2
153.8
40.0
-
37.9
0.3
38.2
40.0
-
29.3
0.3
29.6
40.0
-
150
153
194
78
70
Liabilities & Equity
Accounts Payable
Accrued expenses
Total Current Liabilities
Deferred Rent
Total Liabilities
1.1
10.0
11.1
0.1
11.19
2.5
76.5
79.0
0.1
79.10
2.8
1.5
4.2
0.1
4.35
3.0
51.5
54.5
0.1
54.62
3.3
101.5
104.8
0.1
104.92
3.7
56.5
60.1
0.1
60.26
4.0
56.5
60.5
0.1
60.62
72.6
0.0
8.1
(51.8)
28.9
40
TRUE
387.7
0.0
254.1
(261.7)
380.1
459
TRUE
0.0
254.1
(108.3)
145.9
150
TRUE
0.0
454.1
(355.6)
98.5
153
TRUE
0.0
704.1
(615.2)
88.9
194
TRUE
0.0
804.1
(786.2)
17.9
78
TRUE
0.0
804.1
(795.2)
9.0
70
TRUE
380.2
131.0
(22.0)
(30.9)
Convertible preffered Stock
Common Stock
Additional Paid in capital
Accumulated Deficit
Total Shareholders' Equity
Total Liabilities & Equity
check
Working Capital
Source: Company reports and J.P. Morgan estimates.
16
25.0
68.6
49.0
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
Figure 8: JUNO Cash Flow Statement
Juno Therapeutics Cash Flow Statement ($ millions)
Cory W. Kasimov
cory.w.kasimov@jpmchase.com
212.622.5216
2013A
Non-GAAP Net Income (loss)
Adjustments to reconcile net loss to net operating cash
Depreciation & Amortization
Stock based compensation expense
Non-cash expense in connection with equity issuance
Loss from remeasurement of fair value of convertible stock ooption
Changes in operating assets and liabilities
Prepaid expenses and other assets
Accounts payable
Accrued lia bilities and deferred rent
Cash Flow from Operations
Purchase of PPE
Other
Cash Flow from Investing
Issuance of common stock, net of costs
Issuance of convertible preferred stock, net
Cash Flow from Financing
Total Change in Cash
Beginning Cash Bala nce
Ending Balance: Cash and Investments
$
2014E
2015E
2016E
(112.0) $
2017E
(72.0) $
0.0
0.1
10.2
0.1
5.0
13.0
10.7
$
(0.3)
1.1
10.0
(30.5) $
(7.0)
1.0
(15.0)
(64.3) $
(7.0)
1.0
(205.0)
(304.0) $
(7.4)
1.0
(50.0)
(192.1) $
(7.7)
1.0
(50.0)
(204.3) $
(8.1)
1.0
(120.0)
(210.7) $
(8.5)
1.0
(50.0)
(3.6)
$
(0.04)
(0.0) $
(5.00)
(5.0) $
(20.00)
(20.0) $
(20.00)
(20.0) $
(15.00)
(15.0) $
(5.00)
(5.0) $
(5.00)
(5.0)
$
0.0
66.5
66.5
$
246.0
246.4
492.4
200.0
200.0
$
250.0
250.0
$
100.0
100.0
$
36.0
36.0
$
423.0
36.0
459.0
(12.1)
135.0
122.9 $
30.7
122.9
153.6
$
(115.7)
153.6
37.9 $
$
$
-
5.0
14.3
-
$
(324.0)
459.0
135.0 $
(167.1) $
2019E
(51.8) $
5.0
14.0
-
(155.0) $
2018E
5.0
14.5
-
(103.4) $
5.0
14.8
-
33.9
5.0
15.0
-
$
(8.6)
37.9
29.3
Source: Company reports and J.P. Morgan estimates.
17
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
Juno Therapeutics: Summary of Financials
Income Statement - Annual
Revenues
Cost of products sold
Gross profit
SG&A
R&D
Operating income
EBITDA
Net interest (income) / expense
Other income / (expense)
Income taxes
Net income - GAAP
Net income - recurring
Diluted shares outstanding
EPS - excluding non-recurring
EPS - recurring
Balance Sheet and Cash Flow Data
Cash and cash equivalents
Accounts receivable
Inventories
Other current assets
Current assets
PP&E
Total assets
FY13A FY14E FY15E FY16E
0
0
0
0
0
0
(19)
(37)
(43)
- (108)
(217)
(226)
- (135)
(256)
(269)
- (135)
(256)
(269)
0
0
0
0
0
0
- (135)
(256)
(269)
- (135)
(256)
(269)
7
59
91
93
- (2.28) (2.82) (2.89)
- (2.28) (2.82) (2.89)
FY13A FY14E FY15E FY16E
459
135
123
0
0
0
459
135
123
0
15
30
459
150
153
Total debt
Total liabilities
Shareholders' equity
-
79
380
4
146
55
99
Net income (including charges)
D&A
Change in working capital
Other
Cash flow from operations
-
(72)
5
(21)
24
(64)
(112)
5
(211)
14
(304)
(155)
5
(56)
14
(192)
Capex
(5)
Free cash flow
(69)
Cash flow from investing activities
(5)
Cash flow from financing activities
492
Dividends
Dividend yield
Source: Company reports and J.P. Morgan estimates.
Note: $ in millions (except per-share data).Fiscal year ends Dec
(20)
(324)
(20)
0
-
(20)
(212)
(20)
200
-
18
Income Statement - Quarterly
Revenues
Cost of products sold
Gross profit
SG&A
R&D
Operating income
EBITDA
Net interest (income) / expense
Other income / (expense)
Income taxes
Net income - GAAP
Net income - recurring
Diluted shares outstanding
EPS - excluding non-recurring
EPS - recurring
Ratio Analysis
Sales growth
EBIT growth
EPS growth - recurring
1Q14A
0A
0A
(3)A
(3)A
(8)A
(8)A
0A
0A
(8)A
(8)A
0A
FY13A
-
2Q14A
0A
0A
(5)A
(6)A
(13)A
(13)A
0A
0A
(13)A
(13)A
0A
FY14E
-
3Q14A
0A
0A
(5)A
(13)A
(20)A
(20)A
0A
0A
(20)A
(20)A
0A
FY15E
89.9%
23.8%
4Q14E
0
0
(6)
(86)
(94)
(94)
0
0
(94)
(94)
59
(1.59)
(1.59)
FY16E
5.2%
2.6%
Gross margin
EBIT margin
EBITDA margin
Tax rate
Net margin
-
0.0%
-
0.0%
-
0.0%
-
Net Debt / EBITDA
Net Debt / Capital (book)
-
-
-
-
Return on assets (ROA)
Return on equity (ROE)
- (58.7%)
- (70.9%)
(84.0%)
(97.3%)
(177.5%)
(220.3%)
Enterprise value / sales
Enterprise value / EBITDA
Free cash flow yield
-
NM
(5.8%)
NM
(3.7%)
NM
(1.9%)
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
Analyst Certification: The research analyst(s) denoted by an “AC” on the cover of this report certifies (or, where multiple research
analysts are primarily responsible for this report, the research analyst denoted by an “AC” on the cover or within the document
individually certifies, with respect to each security or issuer that the research analyst covers in this research) that: (1) all of the views
expressed in this report accurately reflect his or her personal views about any and all of the subject securities or issuers; and (2) no part of
any of the research analyst's compensation was, is, or will be directly or indirectly related to the specific recommendations or views
expressed by the research analyst(s) in this report. For all Korea-based research analysts listed on the front cover, they also certify, as per
KOFIA requirements, that their analysis was made in good faith and that the views reflect their own opinion, without undue influence or
intervention.
Important Disclosures
Market Maker: JPMS makes a market in the stock of Juno Therapeutics.
Client: J.P. Morgan currently has, or had within the past 12 months, the following company(ies) as clients: Juno Therapeutics.
Lead or Co-manager: J.P. Morgan acted as lead or co-manager in a public offering of equity and/or debt securities for Juno
Therapeutics within the past 12 months.
Client/Investment Banking: J.P. Morgan currently has, or had within the past 12 months, the following company(ies) as investment
banking clients: Juno Therapeutics.
Investment Banking (past 12 months): J.P. Morgan received in the past 12 months compensation from investment banking Juno
Therapeutics.
Investment Banking (next 3 months): J.P. Morgan expects to receive, or intends to seek, compensation for investment banking
services in the next three months from Juno Therapeutics.
Company-Specific Disclosures: Important disclosures, including price charts and credit opinion history tables, are available for
compendium reports and all J.P. Morgan–covered companies by visiting https://jpmm.com/research/disclosures, calling 1-800-477-0406,
or e-mailing research.disclosure.inquiries@jpmorgan.com with your request. J.P. Morgan’s Strategy, Technical, and Quantitative
Research teams may screen companies not covered by J.P. Morgan. For important disclosures for these companies, please call 1-800-4770406 or e-mail research.disclosure.inquiries@jpmorgan.com.
Juno Therapeutics (JUNO, JUNO US) Price Chart
102
85
68
Price($)
51
34
17
0
Dec
14
Dec
14
Dec
14
Jan
15
Jan
15
Jan
15
Source: Bloomberg and J.P. Morgan; price data adjusted for stock splits and dividends.
The chart(s) show J.P. Morgan's continuing coverage of the stocks; the current analysts may or may not have covered it over the entire
period.
J.P. Morgan ratings or designations: OW = Overweight, N= Neutral, UW = Underweight, NR = Not Rated
Explanation of Equity Research Ratings, Designations and Analyst(s) Coverage Universe:
J.P. Morgan uses the following rating system: Overweight [Over the next six to twelve months, we expect this stock will outperform the
average total return of the stocks in the analyst’s (or the analyst’s team’s) coverage universe.] Neutral [Over the next six to twelve
months, we expect this stock will perform in line with the average total return of the stocks in the analyst’s (or the analyst’s team’s)
19
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
coverage universe.] Underweight [Over the next six to twelve months, we expect this stock will underperform the average total return of
the stocks in the analyst’s (or the analyst’s team’s) coverage universe.] Not Rated (NR): J.P. Morgan has removed the rating and, if
applicable, the price target, for this stock because of either a lack of a sufficient fundamental basis or for legal, regulatory or policy
reasons. The previous rating and, if applicable, the price target, no longer should be relied upon. An NR designation is not a
recommendation or a rating. In our Asia (ex-Australia) and U.K. small- and mid-cap equity research, each stock’s expected total return is
compared to the expected total return of a benchmark country market index, not to those analysts’ coverage universe. If it does not appear
in the Important Disclosures section of this report, the certifying analyst’s coverage universe can be found on J.P. Morgan’s research
website, www.jpmorganmarkets.com.
Coverage Universe: Kasimov, Cory W: ACADIA Pharmaceuticals (ACAD), Acorda Therapeutics Inc. (ACOR), Aegerion
Pharmaceuticals (AEGR), Alkermes, Inc. (ALKS), Amgen Inc (AMGN), Ariad Pharmaceuticals (ARIA), BioMarin Pharmaceuticals
(BMRN), Biogen Idec (BIIB), Celgene (CELG), Clovis Oncology (CLVS), Gilead Sciences (GILD), Incyte Corporation (INCY), Keryx
Biopharmaceuticals (KERX), MannKind Corporation (MNKD), Novavax (NVAX), Otonomy (OTIC), Pharmacyclics, Inc. (PCYC),
Regeneron Pharmaceuticals (REGN), Sage Therapeutics (SAGE), Sangamo BioSciences (SGMO), Seattle Genetics (SGEN), Ultragenyx
(RARE), Vertex Pharmaceuticals (VRTX), ZIOPHARM Oncology (ZIOP), ZS Pharma (ZSPH), bluebird bio (BLUE)
J.P. Morgan Equity Research Ratings Distribution, as of January 1, 2015
J.P. Morgan Global Equity Research Coverage
IB clients*
JPMS Equity Research Coverage
IB clients*
Overweight
(buy)
45%
56%
45%
75%
Neutral
(hold)
43%
49%
48%
67%
Underweight
(sell)
12%
33%
7%
52%
*Percentage of investment banking clients in each rating category.
For purposes only of FINRA/NYSE ratings distribution rules, our Overweight rating falls into a buy rating category; our Neutral rating falls into a hold
rating category; and our Underweight rating falls into a sell rating category. Please note that stocks with an NR designation are not included in the table
above.
Equity Valuation and Risks: For valuation methodology and risks associated with covered companies or price targets for covered
companies, please see the most recent company-specific research report at http://www.jpmorganmarkets.com, contact the primary analyst
or your J.P. Morgan representative, or email research.disclosure.inquiries@jpmorgan.com.
Equity Analysts' Compensation: The equity research analysts responsible for the preparation of this report receive compensation based
upon various factors, including the quality and accuracy of research, client feedback, competitive factors, and overall firm revenues.
Other Disclosures
J.P. Morgan ("JPM") is the global brand name for J.P. Morgan Securities LLC ("JPMS") and its affiliates worldwide. J.P. Morgan Cazenove is a marketing
name for the U.K. investment banking businesses and EMEA cash equities and equity research businesses of JPMorgan Chase & Co. and its subsidiaries.
All research reports made available to clients are simultaneously available on our client website, J.P. Morgan Markets. Not all research content is
redistributed, e-mailed or made available to third-party aggregators. For all research reports available on a particular stock, please contact your sales
representative.
Options related research: If the information contained herein regards options related research, such information is available only to persons who have
received the proper option risk disclosure documents. For a copy of the Option Clearing Corporation's Characteristics and Risks of Standardized Options,
please contact your J.P. Morgan Representative or visit the OCC's website at http://www.optionsclearing.com/publications/risks/riskstoc.pdf
Legal Entities Disclosures
U.S.: JPMS is a member of NYSE, FINRA, SIPC and the NFA. JPMorgan Chase Bank, N.A. is a member of FDIC. U.K.: JPMorgan Chase N.A., London
Branch, is authorised by the Prudential Regulation Authority and is subject to regulation by the Financial Conduct Authority and to limited regulation by
the Prudential Regulation Authority. Details about the extent of our regulation by the Prudential Regulation Authority are available from J.P. Morgan on
request. J.P. Morgan Securities plc (JPMS plc) is a member of the London Stock Exchange and is authorised by the Prudential Regulation Authority and
regulated by the Financial Conduct Authority and the Prudential Regulation Authority. Registered in England & Wales No. 2711006. Registered Office 25
Bank Street, London, E14 5JP. South Africa: J.P. Morgan Equities South Africa Proprietary Limited is a member of the Johannesburg Securities
Exchange and is regulated by the Financial Services Board. Hong Kong: J.P. Morgan Securities (Asia Pacific) Limited (CE number AAJ321) is regulated
by the Hong Kong Monetary Authority and the Securities and Futures Commission in Hong Kong and/or J.P. Morgan Broking (Hong Kong) Limited (CE
number AAB027) is regulated by the Securities and Futures Commission in Hong Kong. Korea: J.P. Morgan Securities (Far East) Ltd, Seoul Branch, is
regulated by the Korea Financial Supervisory Service. Australia: J.P. Morgan Australia Limited (JPMAL) (ABN 52 002 888 011/AFS Licence No:
238188) is regulated by ASIC and J.P. Morgan Securities Australia Limited (JPMSAL) (ABN 61 003 245 234/AFS Licence No: 238066) is regulated by
ASIC and is a Market, Clearing and Settlement Participant of ASX Limited and CHI-X. Taiwan: J.P.Morgan Securities (Taiwan) Limited is a participant
of the Taiwan Stock Exchange (company-type) and regulated by the Taiwan Securities and Futures Bureau. India: J.P. Morgan India Private Limited
(Corporate Identity Number - U67120MH1992FTC068724), having its registered office at J.P. Morgan Tower, Off. C.S.T. Road, Kalina, Santacruz - East,
Mumbai – 400098, is a member of the National Stock Exchange of India Limited (SEBI Registration Number - INB 230675231/INF 230675231/INE
20
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
230675231) and Bombay Stock Exchange Limited (SEBI Registration Number - INB 010675237/INF 010675237) and is regulated by Securities and
Exchange Board of India. Telephone: 91-22-6157 3000, Facsimile: 91-22-6157 3990 and Website: www.jpmipl.com. For non local research reports, this
material is not distributed in India by J.P. Morgan India Private Limited. Thailand: This material is issued and distributed in Thailand by JPMorgan
Securities (Thailand) Ltd., which is a member of the Stock Exchange of Thailand and is regulated by the Ministry of Finance and the Securities and
Exchange Commission and its registered address is 3rd Floor, 20 North Sathorn Road, Silom, Bangrak, Bangkok 10500. Indonesia: PT J.P. Morgan
Securities Indonesia is a member of the Indonesia Stock Exchange and is regulated by the OJK a.k.a. BAPEPAM LK. Philippines: J.P. Morgan Securities
Philippines Inc. is a Trading Participant of the Philippine Stock Exchange and a member of the Securities Clearing Corporation of the Philippines and the
Securities Investor Protection Fund. It is regulated by the Securities and Exchange Commission. Brazil: Banco J.P. Morgan S.A. is regulated by the
Comissao de Valores Mobiliarios (CVM) and by the Central Bank of Brazil. Mexico: J.P. Morgan Casa de Bolsa, S.A. de C.V., J.P. Morgan Grupo
Financiero is a member of the Mexican Stock Exchange and authorized to act as a broker dealer by the National Banking and Securities Exchange
Commission. Singapore: This material is issued and distributed in Singapore by or through J.P. Morgan Securities Singapore Private Limited (JPMSS)
[MCI (P) 199/03/2014 and Co. Reg. No.: 199405335R] which is a member of the Singapore Exchange Securities Trading Limited and is regulated by the
Monetary Authority of Singapore (MAS) and/or JPMorgan Chase Bank, N.A., Singapore branch (JPMCB Singapore) which is regulated by the MAS. This
material is provided in Singapore only to accredited investors, expert investors and institutional investors, as defined in Section 4A of the Securities and
Futures Act, Cap. 289. Recipients of this document are to contact JPMSS or JPMCB Singapore in respect of any matters arising from, or in connection
with, the document. Japan: JPMorgan Securities Japan Co., Ltd. is regulated by the Financial Services Agency in Japan. Malaysia: This material is issued
and distributed in Malaysia by JPMorgan Securities (Malaysia) Sdn Bhd (18146-X) which is a Participating Organization of Bursa Malaysia Berhad and a
holder of Capital Markets Services License issued by the Securities Commission in Malaysia. Pakistan: J. P. Morgan Pakistan Broking (Pvt.) Ltd is a
member of the Karachi Stock Exchange and regulated by the Securities and Exchange Commission of Pakistan. Saudi Arabia: J.P. Morgan Saudi Arabia
Ltd. is authorized by the Capital Market Authority of the Kingdom of Saudi Arabia (CMA) to carry out dealing as an agent, arranging, advising and
custody, with respect to securities business under licence number 35-07079 and its registered address is at 8th Floor, Al-Faisaliyah Tower, King Fahad
Road, P.O. Box 51907, Riyadh 11553, Kingdom of Saudi Arabia. Dubai: JPMorgan Chase Bank, N.A., Dubai Branch is regulated by the Dubai Financial
Services Authority (DFSA) and its registered address is Dubai International Financial Centre - Building 3, Level 7, PO Box 506551, Dubai, UAE.
Country and Region Specific Disclosures
U.K. and European Economic Area (EEA): Unless specified to the contrary, issued and approved for distribution in the U.K. and the EEA by JPMS plc.
Investment research issued by JPMS plc has been prepared in accordance with JPMS plc's policies for managing conflicts of interest arising as a result of
publication and distribution of investment research. Many European regulators require a firm to establish, implement and maintain such a policy. This
report has been issued in the U.K. only to persons of a kind described in Article 19 (5), 38, 47 and 49 of the Financial Services and Markets Act 2000
(Financial Promotion) Order 2005 (all such persons being referred to as "relevant persons"). This document must not be acted on or relied on by persons
who are not relevant persons. Any investment or investment activity to which this document relates is only available to relevant persons and will be
engaged in only with relevant persons. In other EEA countries, the report has been issued to persons regarded as professional investors (or equivalent) in
their home jurisdiction. Australia: This material is issued and distributed by JPMSAL in Australia to "wholesale clients" only. This material does not take
into account the specific investment objectives, financial situation or particular needs of the recipient. The recipient of this material must not distribute it to
any third party or outside Australia without the prior written consent of JPMSAL. For the purposes of this paragraph the term "wholesale client" has the
meaning given in section 761G of the Corporations Act 2001. Germany: This material is distributed in Germany by J.P. Morgan Securities plc, Frankfurt
Branch and J.P.Morgan Chase Bank, N.A., Frankfurt Branch which are regulated by the Bundesanstalt für Finanzdienstleistungsaufsicht. Hong Kong: The
1% ownership disclosure as of the previous month end satisfies the requirements under Paragraph 16.5(a) of the Hong Kong Code of Conduct for Persons
Licensed by or Registered with the Securities and Futures Commission. (For research published within the first ten days of the month, the disclosure may
be based on the month end data from two months prior.) J.P. Morgan Broking (Hong Kong) Limited is the liquidity provider/market maker for derivative
warrants, callable bull bear contracts and stock options listed on the Stock Exchange of Hong Kong Limited. An updated list can be found on HKEx
website: http://www.hkex.com.hk. Japan: There is a risk that a loss may occur due to a change in the price of the shares in the case of share trading, and
that a loss may occur due to the exchange rate in the case of foreign share trading. In the case of share trading, JPMorgan Securities Japan Co., Ltd., will be
receiving a brokerage fee and consumption tax (shouhizei) calculated by multiplying the executed price by the commission rate which was individually
agreed between JPMorgan Securities Japan Co., Ltd., and the customer in advance. Financial Instruments Firms: JPMorgan Securities Japan Co., Ltd.,
Kanto Local Finance Bureau (kinsho) No. 82 Participating Association / Japan Securities Dealers Association, The Financial Futures Association of Japan,
Type II Financial Instruments Firms Association and Japan Investment Advisers Association. Korea: This report may have been edited or contributed to
from time to time by affiliates of J.P. Morgan Securities (Far East) Ltd, Seoul Branch. Singapore: JPMSS and/or its affiliates may have a holding in any of
the securities discussed in this report; for securities where the holding is 1% or greater, the specific holding is disclosed in the Important Disclosures
section above. Taiwan: This material is issued and distributed in Taiwan by J.P. Morgan Securities (Taiwan Limited). India: For private circulation only,
not for sale. Pakistan: For private circulation only, not for sale. New Zealand: This material is issued and distributed by JPMSAL in New Zealand only to
persons whose principal business is the investment of money or who, in the course of and for the purposes of their business, habitually invest money.
JPMSAL does not issue or distribute this material to members of "the public" as determined in accordance with section 3 of the Securities Act 1978. The
recipient of this material must not distribute it to any third party or outside New Zealand without the prior written consent of JPMSAL. Canada: The
information contained herein is not, and under no circumstances is to be construed as, a prospectus, an advertisement, a public offering, an offer to sell
securities described herein, or solicitation of an offer to buy securities described herein, in Canada or any province or territory thereof. Any offer or sale of
the securities described herein in Canada will be made only under an exemption from the requirements to file a prospectus with the relevant Canadian
securities regulators and only by a dealer properly registered under applicable securities laws or, alternatively, pursuant to an exemption from the dealer
registration requirement in the relevant province or territory of Canada in which such offer or sale is made. The information contained herein is under no
circumstances to be construed as investment advice in any province or territory of Canada and is not tailored to the needs of the recipient. To the extent that
the information contained herein references securities of an issuer incorporated, formed or created under the laws of Canada or a province or territory of
Canada, any trades in such securities must be conducted through a dealer registered in Canada. No securities commission or similar regulatory authority in
Canada has reviewed or in any way passed judgment upon these materials, the information contained herein or the merits of the securities described herein,
and any representation to the contrary is an offence. Dubai: This report has been issued to persons regarded as professional clients as defined under the
DFSA rules. Brazil: Ombudsman J.P. Morgan: 0800-7700847 / ouvidoria.jp.morgan@jpmorgan.com.
General: Additional information is available upon request. Information has been obtained from sources believed to be reliable but JPMorgan Chase & Co.
or its affiliates and/or subsidiaries (collectively J.P. Morgan) do not warrant its completeness or accuracy except with respect to any disclosures relative to
21
Cory Kasimov
(1-212) 622-5266
cory.w.kasimov@jpmorgan.com
North America Equity Research
13 January 2015
JPMS and/or its affiliates and the analyst's involvement with the issuer that is the subject of the research. All pricing is as of the close of market for the
securities discussed, unless otherwise stated. Opinions and estimates constitute our judgment as of the date of this material and are subject to change
without notice. Past performance is not indicative of future results. This material is not intended as an offer or solicitation for the purchase or sale of any
financial instrument. The opinions and recommendations herein do not take into account individual client circumstances, objectives, or needs and are not
intended as recommendations of particular securities, financial instruments or strategies to particular clients. The recipient of this report must make its own
independent decisions regarding any securities or financial instruments mentioned herein. JPMS distributes in the U.S. research published by non-U.S.
affiliates and accepts responsibility for its contents. Periodic updates may be provided on companies/industries based on company specific developments or
announcements, market conditions or any other publicly available information. Clients should contact analysts and execute transactions through a J.P.
Morgan subsidiary or affiliate in their home jurisdiction unless governing law permits otherwise.
"Other Disclosures" last revised November 29, 2014.
Copyright 2015 JPMorgan Chase & Co. All rights reserved. This report or any portion hereof may not be reprinted, sold or
redistributed without the written consent of J.P. Morgan. #$J&098$#*P
22








