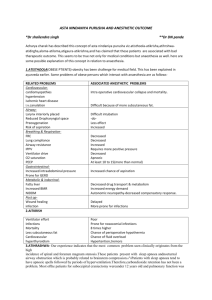
anesthesia guidelines - Tulane University Department of
advertisement

Department of Anesthesiology Obstetric ANESTHESIA GUIDELINES July 2009 INDEX Preface Operating Room Set Up ........................................................................................page 2 Aseptic Technique ..................................................................................................page 2 Anesthesia for Vaginal Delivery..........................................................................pages 3-6 These guidelines for Epidural Block ................................................................................................page 3 Tulane Medical Combined Spinal-Epidural .............................................................................page 5 Hypotension ..........................................................................................................page 6 Center are written with equal weight as Maintenance of Epidural Analgesia ........................................................................page 6 NPO Status in Labor ......................................................................................page 6 PCEA .............................................................................................................page 7 those defined by the Top Ups .................................................................................................................page 8 Policy Statement on TOLAC/Uterine Rupture................................................................................pages 9-10 Anesthesia for Cesarean Section ......................................................................page 10-14 Practice Parameters Regional........................................................................................................page 11 of the American Spinal ...........................................................................................................page 11 Epidural........................................................................................................page 13 Society of Anesthesiologists. General .........................................................................................................page 14 Anesthesia for D&C and D&E ............................................................................page 15 Anesthesia for Postpartum Tubal Ligation......................................................pages 15-16 “Guidelines are Epidural........................................................................................................page 15 recommendations Spinal ...........................................................................................................page 16 General .........................................................................................................page 16 that may identify a Anesthesia for Cerclage Procedure.......................................................................pages 17 particular Nitroglycerin ......................................................................................................pages 18 Wet Taps ..............................................................................................................page 18 management strategy or a range Coagulation and Regional Anesthesia ............................................................pages 20-21 HELLP Syndrome ........................................................................................page 20 Interventional Tocolysis for Fetal Distress.............................................................page 22 of management PCA for Labor Analgesia......................................................................................page 23 strategies.” CPR for the Parturient .........................................................................................page 23 Postpartum Hemorrhage ......................................................................................page 23 Fetal Demise ........................................................................................................page 24 Neuraxial Opioid-Induced Pruritis.......................................................................page 24 Intralipid for Bupivacaine Toxicity ..................................................................page 24-25 References ............................................................................................................page 26 1 Operating Room Set Up All operating rooms on L&D must be clean, organized and left in an assembled fashion after each case. Standard of care dictates that anyone in our department should be able to walk into an L&D OR and be able to easily anesthetize a patient for an emergency cesarean section. The following items are essential for an assembled and organized L&D operating room: ■ A breathing circuit with mask that has been tested for leaks ■ The BP cuff, EKG, and SaO2 cables must be neatly wrapped and ready to use ■ Working laryngoscope blades, 6.0 and 7.0 styletted ET tubes ■ A full, newly labeled syringe of ephedrine and succinylcholine and a secure, readily available induction agent. ■ Working suction with a suction tip attached You are not finished with your case until the OR is put back in shape. The Resident and CRNA assigned to OB are required to check the ORs at the beginning of each day. Aseptic Technique For Neuraxial Anesthesia The importance of using and documenting strict aseptic technique while performing all neuraxial block procedures cannot be overemphasized. Bacterial Meningitis Bacterial meningitis can occur within the same time frame as post dural puncture headache (PDPH), and has been mistaken for PDPH in the past, with catastrophic consequences. It can be lethal in days. Many cases of bacterial meningitis have been linked to poor utilization of aseptic technique in neuraxial anesthesia. For example, in Germany, over a three year period, three cases of bacterial meningitis after spinal or epidural anesthesia were investigated1. In the first two cases, contamination with oropharyngeal flora was highly suspected, but unproven. It was confirmed in the third case, however. Nasal swabs from the operating anesthesiologist, and bacteria obtained from the CSF of the patient were cultured, and the bacteria genetically analyzed. Staphylococcus salivarius, an oral flora, was found to be the causative organism -- and, moreover, was found to be of identical genotypic profile in both the patient and the operating anesthesiologist! This is certainly a sobering case, and it illustrates that we do have the ability to prevent many such disasters by simply being a little more careful with our aseptic technique. Epidural Abscess Epidural abscess is another, though rarer, infectious complication of neuraxial anesthesia – with an incidence of about 1/450,000 (with a much higher incidence where epidural catheters are left in place for more than 72 hours). This entity has a more insidious onset, with progressively increasing back pain over the course of several days a common presentation. Neurological deficits, fever and elevated WBC counts are often later findings, and the treatment is neurosurgical drainage and prolonged intravenous antibiotic therapy2. Use of Cap and Mask None would argue about the importance of sterile prep, drape and sterile gloves, but not all clinicians wear a cap and mask while performing neuraxial procedures. Studies performed by the CDC in 2005, however, concluded that there is sufficient evidence to warrant the wearing of a facemask while placing a catheter or injecting material into the spinal or epidural space3. The article went on to warn about the use of multidose vials for neuraxial anesthesia as an unsafe practice. In addition to a cap and mask, the clinician performing a neuraxial block should wear sterile gloves, and should prep the patient’s back at least twice with either chlorhexidine, chlorhexidine-alcohol, povidone, or povidone-alcohol4. Recent recommendations also suggest that a mask and cap be placed on everyone within 20 feet of provider and patient during epidural placement. The drugs for neuraxial administration should be drawn through the filter needle provided. As a side note, although chlorhexidine has been shown to be a better prep solution, it has not been approved for use in neuraxial anesthesia, as it has not been studied well in this setting. As a result, companies have been reluctant to place it in epidural kits, even though it has been used safely, until further studies are done. This article emphasized again the role of droplet spread oral flora during neuraxial procedures done without a mask. Take care to avoid touching the inside of the catheter hub during re-injections. Some clinicians even take extra care to wear a mask while drawing up medications for re-injection and while administering the reinjection. This would seem wise, as it is difficult to be too cautious in taking steps to avoid such a potentially lethal yet highly preventable complication as bacterial meningitis. 2 Anesthesia for Vaginal Delivery Preparation for Epidural Analgesia 1. Check consent and preoperative forms. If necessary, obtain the consent from the mother with the nurse, spouse and other family members present. Appropriate verbal consent is adequate to proceed with the epidural, but do not forget to document that you obtained a verbal consent prior to the procedure and who was present when this occurred (i.e. nurse and/or family members). Have the consent signed after the patient is comfortable. 2. Co-Load: Each patient should have a working IV in place, and concurrent administration of Lactated Ringers's solution is recommended during epidural placement. 3. 30 cc of cold po Bictria and Reglan 10 mg IV. 4. The epidural cart should be in the room when the epidural is being placed. This makes all the resuscitation drugs and equipment available at the bedside in case of an intravascular local anesthetic injection resulting in seizures or cardiac arrest. or Pepcid 20 mg (or Zantac 50 mg), Monitoring The ASA Guidelines for Regional Anesthesia in Obstetrics (amended by House of Delegates on October 17, 2007) state that "regional anesthesia for labor and/or delivery requires that the parturients’ vital signs and the fetal heart rate be monitored and documented by a qualified individual. Additional monitoring appropriate to the clinical condition of the parturient and the fetus should be employed when indicated. When extensive regional blockade is administered for complicated vaginal delivery, the standards for basic intraoperative monitoring should be applied." In summary, for a laboring patient that delivers spontaneously and receives non-epinephrine test doses, only a non-invasive blood pressure monitor is required. If a patient requires a more extensive block for instrumental or cesarean delivery, standard intraoperative monitoring is required. Test Dose for Epidural Block for Labor The purpose of a test dose is to detect accidental intravascular and subarachnoid injections of local anesthetic. Test dose in the laboring patient is a very controversial topic, with suggestions ranging from an epinephrine local anesthetic mixture, isoproterenol, and air. There is no "perfect" test dose in the laboring mother. Epinephrine has been shown to be an unreliable indicator of intravascular injection in a laboring mother; it can decrease uteroplacental perfusion, and prolong labor when doses greater than 50 µg are administered epidurally. (A 1/200,000 concentration of epinephrine contains 5 µg/ml of epinephrine) Each dose of local anesthetic should be a test dose. Incremental dosing of plain local anesthetic has a long track record of safety. It does not require continuous electrocardiography, decrease uteroplacental perfusion when administered epidurally, or prolong the first stage of labor. Incremental dosing requires that small (3 cc) volumes of local anesthetic be given while asking the parturient for prodromal signs of an intravascular injection of local anesthetic. It is possible that a parturient will not exhibit prodromal signs if an entire induction dose of epidural 0.25% bupivacaine (12 cc) is injected incrementally in an epidural vein. At this point it is important to realize that they will not develop a block either. If a patient does not experience relief with an “appropriate” amount (12 cc) of 0.25% of bupivacaine, do not inject more local anesthetic, as the catheter may be intravascular. We strive for seizure free anesthesia on labor and delivery. In addition to epinephrine not being a reliable intravascular marker in the laboring patient, recent evidence suggests that it may also decrease mobility. Calimaran et al (Anesth Analg 2003;96:1167) used 3 cc of normal saline and 3 cc of 1.5% lidocaine with 1/200,000 epinephrine after CSE and found the test dose cohort had decreased mobility at 60 minutes. Another reason not to use epinephrine. Epinephrine remains an excellent test dose for non-laboring obstetric patients. This would be best applied to elective cesarean section candidates for epidural anesthesia. Summary ■ Test dose in the laboring patient is a very controversial topic. ■ Epinephrine has been shown to be an unreliable indicator of intravascular injection, in part because of the confounding elevations in heart rate that accompany contractions. ■ Each dose of local anesthetic should be a test dose. Incremental dosing with intermittent negative aspiration is essential to safe practice. ■ 2% lidocaine with epinephrine 5 micrograms/mL (1:200,000) remains an excellent choice when dosing an in situ epidural for cesarean delivery. 3 Initiation of Epidural Block for Labor 1. Epidural Block ■ After location of the epidural space, dose with 20 cc of 0.1% plain ropivacaine* and 50 to 100 ug of fentanyl incrementally. Ropivacaine is a safer choice for epidural administration when compared to bupivacaine. In a classic study (Anesth Analg 1989;69:563) human volunteers received intravenous infusions at 10 mg/min to a maximum dose of 150 mg of both bupivacaine and ropivacaine with a minimum of seven days between drugs. Ropivacaine was found to be a safer drug after the following results: 1. Seven of 12 volunteers tolerated the full dose of ropivacaine with one of 12 tolerating the full dose of bupivacaine. 2. The onset of CNS symptoms occurred with mean ropivacaine dose of 60 mg and 40 mg of bupivacaine. 3. Bupivacaine increased the mean values for the PR interval QRS duration and QT interval while ropivacaine produced a change in only the PR internal at five minutes after the infusion was discontinued. *0.1% ropivacaine is mixed with 8 to 9 cc of preservative free normal saline, 1 to 2 cc of fentanyl and 10 cc of 0.2% ropivacaine. Conclusion: Ropivacaine is a less toxic drug than bupivacaine. In a recent edition of Anesthesiology (Dec 2003, vol 99(6)) an editorial and two case reports in non-obstetric patients again demonstrated the safety of ropivacaine. A 66 y/o woman received 6.67 mg/kg and a 66 y/o man received 1.88 mg/kg during attempts at neural blockage. Both patients had successful resuscitation efforts despite the large doses of ropivacaine. A 20 mg dose in a laboring patient is less than 0.33 mg/kg. This is a very safe and effective dose of local anesthetic. The epidural dose recommended in this section is 20 mg of ropivacaine. If this dose was given entirely intravenously accidentally it would be safer than bupivacaine, and depending on the rate of delivery, may not cause any symptoms. If a dose was accidentally given intrathecally, it is an acceptable dose for spinal ropivacaine. In conclusion ropivacaine is less toxic than bupivacaine and if given accidentally intravenously or intrathecally the patient should tolerate the dose without serious sequelae. Safety is the bottom line in choosing ropivacaine over bupivacaine for epidural blockade (Anesth Analg 2003;96:1473). ■ ■ ■ If a patient is not comfortable with a total of 20 cc of 0.1% plain ropivacaine, but has indisputable evidence of an epidural block, an additional 6 cc of 0.2% plain ropivacaine can be injected. If a patient is not comfortable with a total of 20 cc of 0.1% ropivacaine and 6 cc of 0.2% plain ropivacaine within approximately 20 minutes of injection, pull the catheter and start again. 2% lidocaine with 1/200,000 epinephrine, 1.5% lidocaine with 1/200,000 epinephrine and 0.5% bupivacaine are not recommended for use in epidurals for laboring patients. 2.0% plain lidocaine in a volume of 8 to 10 cc that is pH adjusted (1 cc of HCO3- will provide rapid analgesia for a parturient in tumultuous labor). Note --the bevel of the needle should be pointed upwards before catheter placement, and the catheter should be threaded with its natural curvature pointing upwards to help reduce the chance of catheter malposition. Rotation of the needle should be avoided. Although some clinicians favor using saline for LOR, the use of air is very acceptable, and often recommended, and may result in fewer unrecognized dural punctures. The amount of air actually injected into the epidural space should be minimized, as it can increase the risk of a patchy block, presumably by forming bubbles in the LA solution. Overall, however, it is better to use air to reduce the possibility of unrecognized dural puncture. A key to avoiding dural puncture is to advance the needle slowly, millimeter by millimeter! Practically, only a few seconds more are added to the procedure, but these few seconds can help avoid many dural punctures and the potentially devastating consequences of them. The Bromage Technique is a time honored method of holding the epidural needle and syringe, and was developed by Dr. Philip Bromage at McGill University in Montreal. The technique is illustrated below (Bromage, P.R., Epidural Analgesia, 1978, Saunders): Figure 2. Suggested holding of the needle while advancing through the ligamentum flavum. The thumb of one hand applies pressure to the syringe plunger. 4 2. Double Needle Technique for the First and Second Stages of Labor – Combined Spinal-Epidural (CSE) This technique provides nearly immediate pain relief without motor block. This is a perfect for the woman that presents with advanced dilatation and is very uncomfortable. In addition, a recent article (Anesth Analg 2001;93:414) studying predictors of breakthrough pain during labor found that “the combined spinal / epidural technique may be superior to conventional epidural anesthesia, because breakthrough pain occurred less often.” The combination of CSE and PCEA is the best approach to labor analgesia and decreased anesthesia manpower requirements. ■ Prior to the block, obtain 2cc of fentanyl and a 30 cc vial of 0.25% bupivacaine from the Pyxis machine. ■ Open and place the fentanyl and bupivacaine on the worktable prior to placing the epidural. ■ After location of the epidural space, place a 5” 27 gauge pencil point whitacre spinal needle (located in the epidural cart) through the epidural needle until you feel the “pop” of the spinal needle through the dura mater. Warn the patient that they may experience a paresthesia at this point. ■ If you do not get CSF return, you may not be in the epidural space or the epidural needle is in a lateral part of the epidural space. Inject 10 µg of fentanyl and 1 cc of 0.25% bupivacaine with a 3 cc syringe through the spinal needle. In a recent study (Anesthesiology 2001;94:593) the optimal dose of intrathecal fentanyl was between 5 µg and 15 µg. The reduced dose of fentanyl decreased pruritus almost in half (73% to 40%). The duration of the intrathecal dose was approximately 60 minutes. ■ Remove the spinal needle, inject the remainder of the PFNS through the epidural needle and thread the epidural catheter. ■ You should not have to dose any epidural local anesthetic via the epidural catheter. ■ Begin the epidural PCEA maintenance infusion immediately. Pruritis can occur (please see section on Neuraxial OpioidInduced Pruritis in these Guidelines). ■ Have ephedrine readily available after CSE placement, as parturients can experience a significant drop in blood pressure. CSE should not be considered in situations of fetal distress (a threatening or ominous fetal heart tracing, thick meconium, etc.), in patients with fetal disease (cardiac for example), or premature infants. (BJOG 2002;109:274-81) CSE Dose for Labor Analgesia* 1 cc (2.5 mg) of 0.25% bupivacaine and fentanyl 10 µg (0.2 cc) *Excellent choice for: (1) the very uncomfortable parturient with an advanced dilitation (>7 cm), (2) the multiparous parturient at 4-5 cm with an anticipated delivery within a couple of hours or (3) almost any parturient, even very early in labor, for reasons explained below. Advantages and Uses of CSE: There are many advantages and uses for the CSE, and it has shown itself to be a very fast, safe and effective technique, with wideranging applications. Once only reserved for parturients very late in labor or who are very uncomfortable, it is now commonly being used routinely for practically all parturients, even very early in labor, for reasons of its’ many advantages -- which extend beyond rapid onset of analgesia -- which we will examine in this section. Additionally, it may be used for Cesarean section, where a surgical spinal dose may be given, conferring the benefit of the dense block associated with a spinal anesthetic, while allowing placement of an epidural catheter, which can be left de novo, to be used in case the operation takes longer than anticipated, and block regression takes place. This is especially useful if the section is anticipated to be more difficult, such as a patient s/p multiple repeat sections. These patients often have much scar tissue which makes it more difficult surgically. One may ask of the above use of CSE, why not simply proceed with a straight spinal there? Even with long acting agents and vasoconstrictors, a complicated case can outlast the spinal. What about a straight lumbar epidural? There wouldn’t be a problem with that at all, however, it can still be argued that CSE can be advantageous in that case. A surgical-level block is established more quickly, and a spinal block tends to be denser and more evenly distributed than a straight epidural block. Furthermore, the time required for placement of a CSE is not significantly increased over a straight epidural. In fact, perhaps the only reasons it wouldn’t be better would be if the risks/side effects were greater for CSE, or it were a case where a spinal anesthetic is best avoided. Let us explore these considerations. Some clinicians are concerned that CSE carries a higher incidence of side effects and complications, no matter what setting it is used in. They are worried that there is more motor block, higher risk of dural puncture headache and high spinal, and higher incidence of failed epidural block after the spinal wears off. Some even believe it carries a greater risk of C-section. Fortunately, studies have shown these fears to be essentially unfounded. As a matter of fact, studies have shown the opposite. The overall incidence of failure, inadequate epidural analgesia, catheter replacement, intravenous epidural catheter, and dural puncture and PDPH were all decreased with CSE!1 5 Note – a 27 G, long spinal needle should ideally be used for CSE, not a 25 G, which could confer a greater risk of PDPH (about 1% with the 25 G, compared to possibly as low as 0.1% for the 27 G). It must be remembered that a pencil-point needle (ie. Sprotte or Whitacre) should almost always be selected, while all cutting needles, (ie. Quincke), should be avoided for both CSE and straight spinals in parturients, due to the substantially increased risk of PDPH with the cutting needles. It must be emphasized that CSE has time and time again shown advantage for use in laboring parturients. In comparison with straight lumbar epidurals, fetal, neonatal and maternal side effects are not increased, and the risk of c-section is not increased.2 Fetal bradycardia may be associated with the CSE technique. However, the mode of delivery is unchanged when compared to epidural analgesia for labor. Specifically, the C/S rate is not higher in the CSE group. Motor block and pruritis may be increased, but usually only transiently, and can be reduced by combining an opioid with a local anesthetic, thereby reducing the total dose-requirement of each, through synergistic effects. A study has shown that the optimum dose of intrathecal fentanyl/bupivacaine to yield the best efficacy to side effect ratio is Bupivacaine 2.5 mg, and fentanyl 10 to 15 mcg.3 Ropivacaine, although off-label for spinal use, has a long track record of intrathecal use, and can be used in the same dose proportion as bupivacaine in the CSE, and has the benefit of less motor block.4 When placing a CSE, one should not dose the catheter with a large bolus of local anesthetic soon afterwards (less than five minutes), as a high spinal may result. The reason for this is not diffusion through the dural rent, as some believed previously, but simply because a large bolus increases the pressure in the epidural space, and that pressure is transmitted to the subarachnoid space, pushing up the level. It should not be necessary to dose an epidural catheter at all after CSE, assuming the appropriate spinal dose has been administered. Also note that the incidence of intrathecal catheter is not increased in CSE. Studies have shown that the catheter doesn’t work its’ way through the tiny “dural rent” of the CSE. One may ask why the incidence of failed epidural, unilateral block and dural puncture are reduced with CSE. The answer is quite simple. In order to get a good flow of CSF, the epidural needle must be in the epidural space and not a false space, and it must be fairly close to a perfect midline insertion point, reducing the risk of a failed or unilateral block. Finally, it reduces the incidence of wet tap because it allows the clinician to test if they are at a false loss of resistance by placing the spinal needle and looking for CSF after the loss of resistance, rather than advancing forward blindly. Thus, the spinal needle becomes like a “sounding device.” CSE should not be considered in fetal distress, severe pre-eclampsia (controversial), aortic stenosis, idiopathic pulmonary hypertension, severe cardiomyopathy and right to left cardiac shunts, for example. Note that in some of those above conditions, a straight epidural should perhaps be avoided too. So, the evidence is overwhelming. CSE is the best technique for labor analgesia and other uses such as anticipated difficult csection, for multiple reasons which extend well beyond quick onset. It is well supported to use the technique in practically all laboring patients, in early or late labor, except in the few cases where a spinal should be avoided. For such a relatively simple technique, the benefits of CSE are tremendous. Hypotension All laboring patients require uterine displacement after initiation of epidural blockade for the duration of labor and delivery. If recurrent hypotension occurs during labor after an adequate fluid bolus and with a normal sensory block level, make sure there is left uterine displacement. IM ephedrine is almost never indicated to maintain maternal blood pressure if preload, sensory block and positioning are properly done. Maintenance of Epidural Analgesia of Labor Maintenance PCEA infusions should be assessed every 2 to 3 hours. This assessment is necessary to ensure patient comfort, uniformity of block, and the proper infusion rate and to rule out intravascular or subarachnoid catheter migration (i.e. a lack of block or an extensive block with recurrent hypotension, respectively). Each assessment should be documented on the anesthesia record with a recent set of vital signs, the level of sensory blockade, and level of patient comfort. It is essential that any problems with labor epidurals are found out and fixed. An epidural may be needed for a STAT delivery at any time. A labor epidural that requires frequent top-ups may be due to a poorly placed catheter or a patient that is experiencing painful dysfunctional labor. Be sure that your epidural catheter is working in the event that it is needed for a cesarean section. If you are in doubt replace the catheter (Anes April 1999; SOAP supp, A41). NPO status in labor has evolved from a strict NPO status to ice chips commonly available to parturients. A recent study (Anesth Analg 2002;94:404-8) evaluated the metabolic effects of allowing women isotonic sports drinks (Gatorade) during labor. The results 6 supported a reduction of maternal ketosis in labor without increasing gastric volume. The incidence of vomiting and the volume vomited, maternal and neonatal outcome during labor were similar. The premixed solution for maintenance of labor analgesia is supplied in the Pyxis machine. Patient controlled epidural analgesia (PCEA) Patient controlled epidural analgesia (PCEA) is popular because of high maternal satisfaction and the dose sparing effect with local anesthetic and narcotic. A recent meta analysis (Anes 2001; v94:A71) of PCEA versus continuous infusion again shows that PCEA reduces the number of clinical interventions compared to continuous infusions and reduce the local anesthetic dose to the laboring patient. In addition, there was a strong trend toward reduced motor block. ■ Program the epidural infusion pumps with a basal rate of 6 cc/hr and a bolus of 6 cc with a 15 minute interval and a 30cc 1 hour limit. The directions are labeled on each pump on L&D. The basal rate with a bolus will reduce the need for top-ups. (Anesth Analg 1994; 79:80) ■ Explain to the patient that they will be dosing their epidural. All they have to do is push the button when they begin to feel pain. ■ Assure the patient that they can always call us for questions or a “top up.” This is the preferred technique for epidural maintenance. Figure 1. Area of diffusion of the contrast agent during continuous and intermittent infusions. The above diagram illustrates that one of the mechanisms of the superior efficacy of the intermittent dosing of epidural catheters (ie. by PCEA), is due to the improved spread/flow characteristicsof local anesthetic given by bolus injection. (Diagram from A. Murat Kaynar, K.B. Shankar, Epidural Infusion: Continuous or Bolus?, Anesth Analg 1999; 89:534). 7 Basic Concepts: Epidural Top-ups ■ ■ ■ ■ ■ If a parturient with a labor epidural complains of discomfort, consider having them examined to make sure that delivery is not imminent, and check that the epidural catheter is still taped in proper position, and at the proper depth. It may be valuable to check the epidural catheter and tubing connections and make sure the pump is on. Check the patient’s level bilaterally with an alcohol swab (not a needle). They shouldn’t feel the coldness of the swab if the (pain and temperature) fibers are blocked by the epidural. Next, feel the patients’ feet and lower legs – if one side is cooler, that can mean that the anesthetic is not distributing on that side. The sympathectomy induced by the epidural should cause lower extremity vasodilation, leading to warm feet bilaterally. If one side is cooler, turn the patient with the cooler side down, to allow gravity to help distribute the anesthetic, and then administer the topup dose. Determinants: ■ Even though gravity is a minor determinant of epidural level, it still has some role to play in it. ■ The major determinant for epidural level is volume. ■ The major determinant for depth of epidural block is the drug dose (concentration). ■ Every epidural dose should be considered a test dose, and preceded by aspiration. Catheter migration can occur, and a catheter can become intravascular, or even erode through the dura and become intrathecal. Dosing the catheter in those cases can be lethal. Chloroprociane: ■ Chloroprocaine is not a good choice for top ups. It will result in excessive motor block and antagonize the action of bupivacaine and neuraxial µ-agonists (fentanyl). ■ Chloroprocaine should be used when delivery is imminent for a perineal dose or for cesarean section. Top Up Dose ■ ■ Initial Top Up: 0.125% bupivacaine or 0.1% ropivacaine 6 cc, may repeat if the level T10 or rising 2nd Bullet: Inadequate Analgesia despite bilateral T10 level with the dosing described above: 6 cc of 0.25% Always strive to administer the smallest effective dose of local anesthetic to achieve patient comfort and mobility. Perineal Dose When a perineal dose is requested, find out what the obstetric plan is for delivery. For a spontaneous delivery, the goal is to maintain the maternal pushing effort, while providing perineal anesthesia for cutting and repair of the episiotomy. Deliver this dose with the mother in the sitting position. Spontaneous Delivery 0.25% bupivacaine or 0.2% ropivacaine 6 to 8 cc If an instrumental delivery is anticipated, a denser sacral block is required. Instrumental Delivery 3% chloroprocaine (alkalinized*) 6 to 8 cc OR 2.0% lidocaine (alkalinized*) 6 to 8 cc *1 cc of sodium bicarbonate per 10 cc of local anesthetic 8 Trial of labor after Cesarean Delivery (TOLAC) Standards of Care: There is an evolving recommendation regarding "immediate" availability of the obstetrician and anesthesiologist during the active phase of labor for TOLAC / VBAC. "A management plan for uterine rupture and other potential emergencies requiring rapid cesarean section should be available and documented for each woman undergoing TOLAC." This is a recommendation from the American Academy of Family Physicians in 2005. The 2004 ACOG guidelines require immediate availability. At Tulane we must be on alert and be aware when a TOLAC patient is on the unit. If an Obstetrician is in-house we should be in-house also. Uterine Rupture: The incidence of uterine rupture during trial of labor varies between 1% and 3.7%. Serious maternal and fetal morbidity or mortality occurs in 10-25% of cases of uterine rupture. The 2004 ACOG practice bulletin on (TOLAC) and The Joint Commission on Accreditation of Healthcare Organizations standards now state that because uterine rupture may be catastrophic, (TOLAC) should be attempted in institutions equipped to respond to emergencies with physicians immediately available to provide emergency care. The term immediately has replaced readily available. However, the definition of immediate availability of personnel and facilities remains a local decision based on each institution's available resources and geographic location. Signs and Symptoms of Uterine Rupture: Uterine rupture refers to a separation of a uterine scar that is clinically apparent and results in fetal distress and maternal hemorrhage requiring emergency cesarean delivery or postpartum laparotomy. Although the diagnosis may be difficult because of the variable presentation and degree of scar separation, fetal bradycardia is present in nearly 70% of cases and may be preceded by a nonreassuring fetal heart rate tracing. Fetal bradycardia is the most common sign of uterine rupture. Other signs and symptoms include: ■ Abdominal pain (7-10%) ■ Vaginal bleeding (3-5%) ■ Hemodynamic instability (5-10%) ■ Recession of presenting part (< 5%) Although pain is not often associated with uterine rupture, some patients will complain of varying and/or upper abdominal pain resulting from blood and amniotic fluid produced by diaphragmatic irritation. The classic symptoms (i.e., hypotension, fetal 9 bradycardia, loss of contractions measured by intrauterine pressure catheterization, atypical abdominal pain, and vaginal bleeding) are present in only 17% of cases. Uterine dehiscence refers to a subclinical separation of a previous uterine incision and is often, but not always, asymptomatic. Although transverse uterine scars are assumed to be safer and less vascular than classic uterine scars, delayed diagnosis and treatment of transverse scar rupture can still result in serious maternal and fetal complications. Anesthetic Management of (TOLAC): The preponderance of evidence suggests that labor epidural analgesia may be used safely during a trial of labor and does not affect the likelihood of successful (TOLAC). Success rates for (TOLAC) are similar in women who do and do not receive epidural analgesia, as well as in those women who receive other types of pain relief. Adequate pain relief may also encourage more women to choose trial of labor. A functional epidural catheter can facilitate transition to surgical anesthesia if time allows and cesarean delivery or uterine exploration become necessary. The use of concentrated local anesthetics (e.g., 2% lidocaine, 2-3% 2-chloroprocaine) during labor may mask the breakthrough pain of uterine rupture, but epidural analgesia rarely masks the signs and symptoms of uterine rupture when diluted concentrations of local anesthetic with or without opioids are administered. Breakthrough or varying pain during trial of labor may be indicative of uterine rupture and should be evaluated carefully (ACOG Practice Bulletin 2004). Management of Uterine Rupture: Obstetric management of frank uterine rupture includes immediate laparotomy and cesarean delivery. Because uterine atony often follows uterine rupture, ligation of the uterine, ovarian, and hypogastric arteries can be helpful in reducing blood loss and preventing hysterectomy. Choice of anesthetic depends on maternal hemodynamic status, fetal status, amount of expected blood loss, and risk of cesarean hysterectomy. Either regional or general anesthesia is an acceptable anesthetic choice if the mother and fetus are stable. In parturients with uncertain intravascular volume, spinal or epidural anesthesia may be unsafe because the sympathetic blockade produced by spinal or epidural anesthesia can impair the patient's ability to respond to hemorrhage. Fetal distress increases the urgency of delivery and general anesthesia may be necessary, especially in the absence of a preexisting epidural catheter. (Anesthesiology 2003;99:1444) Anesthesia for Cesarean Section Regional Anesthesia: Regional anesthesia is an excellent choice in cesarean section for fetal distress. There is less risk to the mother and neonate and higher maternal satisfaction with regional anesthesia. Spinal Anesthesia: Spinal anesthesia should be the first choice for cesarean section. Spinal anesthesia requires less time to induce, provides a more comfortable and reliable block for the mother, has the same incidence of postdural puncture headache (PDPH) as epidural anesthesia when a 25-gauge Whitacre needle is used, and eliminates the risk of local anesthetic toxicity. Epidural or Combined Spinal-Epidural Anesthesia: Epidural or Combined Spinal-Epidural anesthesia is indicated for cesarean section when the duration of the operation is anticipated to last greater than 90 minutes. Use the epidural catheter in place for labor analgesia for cesarean section. Do not convert a labor epidural or poor epidural block to a spinal anesthetic without dramatically decreasing the spinal dose because of the great risk of a high spinal (see formula in Spinal Anesthesia for C/S). General Anesthesia: General anesthesia is indicated when the speed of induction is of the utmost importance in a patient with an "acceptable" airway, for maternal hypovolemia or refusal of regional anesthesia. General anesthesia, for any reason, requires confidence in the anesthesiologist's ability to secure the maternal airway and expert knowledge of a drill to manage a difficult airway. 10 Regional Anesthesia Preparation for Regional Anesthesia: 1. Obtain and/or review the preoperative evaluation and consent. Discuss with the obstetrician the indication for the cesarean section. Knowledge of the obstetrical plan will always help your ability to deliver the optimal anesthetic for each delivery. Decide on postoperative analgesia (discussed below). 2. Place an 18 gauge IV. 3. Bictria 30 ml PO, Famotidine (or Ranitidine 50 mg IV), and Reglan 10 mg slow IVP. (on the standing orders for cesarean section) Monitoring Regional Anesthesia: Standard intraoperative monitoring is required including fetal heart rate before and after the administration of neuraxial blockade (ASA Practice Guidelines for Obstetric Anesthesia, 2007). Additional monitoring appropriate to the clinical condition of the parturient and fetus should be employed when indicated. Spinal Anesthesia Preparation for Spinal Anesthesia: Make sure the surgeon is in house or “really close” and ready before placement of the spinal. For an incision time of 07:30 you should have the patient positioned on the OR table by no later than 07:15. If difficulty with the spinal is anticipated or a doubleneedle technique is going to be done, get in the OR earlier. Spinal anesthesia is an acceptable choice for anesthesia in the severe preeclamptic patient providing coagulation studies are acceptable. Recent articles (Anesth Analg 2003; 97:621, 867) again support the use of spinal anesthesia in severe preeclamptic patients. In fact, Aya et al suggest that the risk of hypotension is significantly less in severely preeclamptic patients compared to healthy patients. 1. Co-Load: Each patient should have a working IV in place, and concurrent administration of Lactated Ringers's solution is recommended during spinal anesthesia. 2. The spinal anesthesia kits provided on labor and delivery contain a 25-gauge Whitacre needle and hyperbaric bupivacaine. You may instead use a 27 G Whitacre (packaged as single needles, slightly lower risk of PDPH – possibly as low as 0.1% compared to 1% for the 25 G). 3. Position the mother in the right lateral decubitus or sitting position for placement of the spinal block. After the spinal is finished immediately turn the mother supine with left uterine displacement. Left uterine displacement is easily accomplished with a 1 liter fluid bag under the mother’s right hip under the OR mattress. This makes it easy to pull out the roll after delivery, which helps the obstetricians operate more comfortably. A recent article regarding aortocaval compression (Anesth Analg 2003;97:256) was unable to demonstrate any additional benefit of lateral table tilt once the patient was in a left lateral tilt position. It is essential that the uterus is actually displaced laterally (feel the uterus). The act of placing a roll under the right hip alone is not adequate. You must assure left uterine displacement. 4. HYPOTENSION will occur in patients that receive spinal anesthesia. To reduce the incidence of hypotension a rapid adequate co-load and LUD are essential. Prophylactic ephedrine (10 mg IVP) may be helpful if given IVP at the time of intrathecal injection. Prophylactic IM ephedrine is neither reliable nor effective in reducing the incidence of maternal hypotension during cesarean section. Check maternal blood pressure every 2-2.5 minutes after the spinal is placed until delivery. After delivery, decrease the frequency to every 5 minutes. During the procedure maternal hypotension or maternal nausea should be treated aggressively with 10 mg of IVP ephedrine or phenylephrine 40 µg IVP. A good rule of thumb is to use ephedrine if the heart rate is below 80 and use phenylephrine if the heart rate is above 110. The pressor can be used in combination. Use clinical judgement. Phenylephrine is an effective and safe vasopressor in patients without uteroplacental insufficiency (J Clin Anesth 1991; 3:301). A review of ephedrine vs. phenylephrine (Anesth Analg 2002; 94:920) does not 11 support the traditional idea that ephedrine is the preferred choice for the management of maternal hypotension during spinal anesthesia for elective cesarean delivery in healthy patients. The women treated with phenylephrine had neonates with higher umbilical arterial pH values than those given ephedrine. Note that maternal heart rate will decrease with bolus phenylephrine significantly more than in the non-pregnant patient. Have some glycopyrolate or atropine available. 5. SPINAL DOSING: Spinal dosing for the average person (4' 10" to 5' 10") is independent of height. Baricity and position will determine the number of dermatomes blocked. For extremes of height the dose of spinal bupivacaine (0.75% bupivacaine and 8.25% dextrose) can be altered. Fentanyl is an excellent adjunct to spinal bupivacaine for cesarean section. A 10 µg (0.2 cc) dose of fentanyl immediately improves intraoperative patient comfort with minimal side effects. Fentanyl should be included in the spinal solution even in patients selected to receive spinal morphine for post operative pain. The dose of Duramorph (preservative free spinal morphine) should be included in the spinal solution dose when it is chosen as the agent for postoperative pain control (see below). A double-blinded randomized comparison of 100 μg intrathecal, 200 μg intrathecal and 3 mg of epidural duramorph found a decrease incidence of itching with the 100 μg dose. (Anesth Analg 2002;95:436) Cesarean Section Spinal Solution* Spinal Bupivacaine - 10 mg (1.3 cc) to 12 mg (1.6 cc) or Spinal Lidocaine 60 mg (4cc) Fentanyl - 10 µg (0.2 cc) Duramorph - 100 µg to 200 μg (0.2 cc to 0.4cc of 5mg per 10cc Duramorph) [If patient < 4' 10" decrease dose of spinal bupivacaine to 9 mg (1.2 cc) and if patient > 5' 10" increase dose to 15 mg (2 cc)] *Check out of the pyxis under spinal c/s kit A 12mg spinal bupivacaine dose should provide 90 minutes of anesthesia from the time the spinal is given. Spinal lidocaine will provide 45 to 60 minutes of anesthesia in the dose range above. If increased duration is required because of a longer than normal anticipated cesarean section time, there are four good options: 1. Increase the dose of spinal bupivacaine to 15 mg (2.0 cc). This should provide an additional 15 to 20 minutes. 2. Add 0.2 mg (0.2 cc) of epinephrine to the spinal solution. This will provide an additional 15 to 20 minutes and increase the density of the spinal block. However, epinephrine will result in a prolonged lower extremity motor block, which will increase recovery time. This is not a great choice. 3. A double-needle technique can be used. Inject the spinal bupivacaine solution subarachnoid and then have an epidural catheter available if the case is prolonged. It is wise to inject 15 mg of spinal bupivacaine when utilizing the double needle technique. 4. Abandon the spinal and use an epidural technique. Spinal Anesthesia for Cesarean Section after Failed Labor Epidural: It is always awkward situation when you dose up a labor epidural for cesarean section and the surgical block does not materialize. What to do? You may not have time to resite the catheter and you may be worried about local anesthetic toxicity. If you pull the catheter and proceed with a spinal you are at a documented risk for a total spinal and a whole new set of problems. Two recent abstracts presented at SOAP 2002 (Anes April 2002, P-2, and P-68) provide some resolution to this problem. Resolution: 1. 2. Dose the spinal with 1.2 cc (10 mg) of spinal bupivacaine (0.75% bupivacaine and 8.25% dextrose) in the sitting position. Let the patient sit for 2 minutes then place supine with LUD. Out of 106 cases none resulted in a high spinal. Use the formula: Dose =[ # of segments with no block + (# of segments with patchy block x 0.5)]x 12 mg 18 The total number of segments required for a cesarean section block is 18: 5 sacral, 5 lumbar, and 8 thoracic. Count up the number of segments with inadequate block and multiply x 0.5, then add the number of segments with no block and multiply the sum by 12 and divide the product by 18 and you have your spinal dose. 12 Solution: The solution to this problem is to identify poor epidurals when the patient is laboring. Do not hesitate to replace a poorly functioning epidural on a laboring patient. It is always the correct thing to do. The patient will be appreciative that you are working to make them comfortable. Don’t forget that patients with painful dysfunctional labor are at high risk for cesarean section. Epidural Anesthesia Preparation for Epidural Anesthesia: 1. In order for a 7:30 cesarean section incision time you should be in the OR with the patient on the table by 7:00. If you anticipate a difficult epidural, start earlier. 2. Co-Load: Each patient should have a working IV in place, and concurrent administration of Lactated Ringers's solution is recommended during epidural placement. 3. Left uterine displacement 4. Agents for cesarean section: 2% lidocaine 2% lidociane in a volume of 20 cc provides surgical anesthesia for 60 minutes. A top up of approximately 10 ml is required at 60 minutes (the time of 2 dermatome regression, typically from T4 to T6) for increased duration of surgical anesthesia. The onset time will be decreased and the block made denser with the addition 1 ml of sodium bicarbonate to a 10 cc volume of lidocaine. The addition of 100-µg fentanyl to the epidural solution will improve the intensity of the block and patient comfort. Lidocaine Epidural Cesarean Section Dose 20 cc of 2% lidocaine 2 cc of sodium bicarbonate* 2 cc of fentanyl 3-4 mg of Duramorph for post-op pain *2% lidocaine and sodium bicarbonate are available in the Pyxis on L&D, select the epidural c/s kit 3% 2-chloroprocaine 3% 2-chloroprocaine in a volume of 20 to 25 cc provides rapid dense surgical anesthesia for approximately 45 minutes. Additional top-up doses of 10 cc (one-half the original dose) are required every 30 minutes for continuation of surgical anesthesia. It is very important not to forget to top-up 3% 2-chloroprocaine because the onset of patient discomfort is rapid, and it is difficult to get them comfortable again. 3% chloroprocaine has the shortest half-life of any local anesthetic (23 seconds maternal, 45 seconds fetal). Because of the short half-life, its rapid onset, lack of fetal ion trapping, and dense block, it is the local anesthetic of choice for a delivery when the fetus may be acidotic (i.e. fetal distress). The onset time is decreased by the addition of 1 cc of sodium bicarbonate to 10 cc of 3% 2-chloroprocaine. 3% 2-chloroprocaine behaves as an epidural µ-antagonist. Because of this unusual property epidural fentanyl will not be effective in augmenting an epidural block and the duration of epidural morphine can be shortened. However, 4 mg dose of duramorph seems to work for post-op pain control. The last important property of 3% 2-chloroprocaine is the ability for excellent tissue diffusion. This is clinically useful in the circumstance when an epidural block with 2% lidocaine for cesarean section is "spotty," despite an adequate volume of local anesthetic and the addition of epidural fentanyl. 10 cc of alkalinized 3% 2-chloroprocaine will often save the block and prevent the use of general anesthesia. 3% 2-chloroprocaine Epidural Cesarean Section Dose 20 to 25 cc of 3% 2-chloroprocaine 2 to 3 cc of sodium bicarbonate 4 mg of Duramorph for post-op pain (DON'T FORGET TO TOP-UP AT 30 MINUTES) 13 Cesarean section for a laboring patient with an existing epidural: Cesarean section for a laboring patient with an existing epidural occurs commonly in our practice. Prepare the patient as stated above with the proper monitoring and aspiration prophylaxis. A patient that already has a sympathectomy from the labor epidural usually requires very little (500 cc) or no additional preload. The best policy is to titrate IV lactated Ringer's solution as dictated by maternal hemodynamics. The dose of local anesthetic typically does not differ from the dose used for an elective cesarean section. For example, a cesarean section indicated for a first stage arrest of dilitation, where the patient has an existing T10 level, usually requires the full 20 cc dose of 2% lidocaine. The best policy is to always treat each dose as a test dose and measure the response of your local anesthetic in each individual patient. The use of an epinephrine test dose, 3 cc of 1.5% lidocaine with epinephrine 1/200,000, is appropriate in a laboring patient that is comfortable with a working epidural. Each LDR room is equipped with full monitoring and is the ideal place to establish a surgical block for cesarean section. Establishing your block for cesarean section in the LDR offers the advantage of the catheter not being accidentally displaced during transport before local anesthetic is given and your block sets up during transport to the OR. This saves time. Don’t forget to test dose the catheter for possible intravascular migration. General Anesthesia Preparation for General Aneshesia: 1. Airway Management and Pre-Oxygenation: Proper airway management and pre-oxygenation for 2 minutes at a regular respiratory rate is vital. The time for preoxygenation may be reduced by nearly one-half if maternal respiratory rate is increased. All inductions for general anesthesia in pregnancy are rapid sequence inductions. It may be beneficial to "ramp" the patient, especially obese patients, with shoulder rolls to optimize the alignment of the oral, pharyngeal, and laryngeal axes. IV Induction Agents: Thiopental 4 mg/kg or Propofol 2-2.5 mg/kg (based on pregnant body weight) Ketamine 1 mg/kg (pbw) - indicated for hypovolemic, hypotensive or asthmatic q patients Succinylcholine 1.5 mg/kg (pbw) The goal during induction of general anesthesia for cesarean section is to optimize conditions for the mother primarily, and the fetus secondarily. Don't forget that nearly one-half of all reported anesthetic morbidity in pregnant women in the US involves airway mismanagement. Do not proceed with induction unless you are sure you can intubate the patient. 2. 3. Maintenance of general anesthesia: A Fi02 of at least 0.5 must be maintained until delivery. The use of volatile anesthetics has been considered controversial because of their ability to decrease uterine tone. Obstetricians have implicated the use of volatile anesthetics during cesarean section with increased blood loss because of decreased uterine tone. Fortunately, excellent prospective work has shown that when the decreased MAC associated with pregnancy is taken into account and appropriate concentrations of volatile anesthetics are used there is not increased blood loss when compared to epidural anesthesia. In addition, without the use of a volatile anesthetic during cesarean section the incidence of maternal recall is nearly 25%. This is bad, the use of a BIS monitor should be considered. The message is use volatile anesthetics in appropriate concentrations for pregnancy and pay attention to uterine tone (give the pitocin as soon as the cord is clamped, 3 to 5 units IVP and 20 units per liter of IV fluids). Before delivery: 50% nitrous oxide or 100% 02 50% oxygen Isoflurane, Desflurance or Sevoflurane at 1/2 MAC, titrate to optimize maternal hemodynamics After delivery: 70% nitrous oxide 30% oxygen Fentanyl 2 to 4 cc IVP Midazolam 2 to 4 mg IVP Isoflurane, decrease until adequate uterine tone is established, then increase as tolerated Morphine, titrated on emergence to optimize maternal comfort PCA pump for postoperative pain control 14 Anesthesia for D&C and D&E Procedures A D&C (dilation and curettage) is a very common procedure on labor and delivery. The anesthetic options include a general anesthesia or a saddle block. Controversy exists surrounding general anesthesia focusing on the risk of maternal aspiration. Some anesthesiologist feel that every D&C should be intubated because important hormonal factors (progesterone, motilin and gastrin) exist early in pregnancy that are known to increase maternal gastric volume and reduce gastro-esophageal sphincter tone. However, there is a historical reference for early (< 8 wga), elective, slender body habitus patients to have a D&C done successfully by mask anesthesia. Women < 8 wga can be masked or LMA if there are no other contraindications (body habitus, NPO status, etc.), if > 8 wga consider rapid sequence induction and endotracheal intubation. A D&E (dilation and evacuation) is a procedure to empty the uterus in women more advanced in their pregnancy (late 1st or early 2nd trimester). Because these women are further along in their pregnancy endotracheal intubation can be considered safer than mask anesthesia. There is also a greater risk of maternal blood loss with a D&E. A large bore IV and a preoperative type and screen is recommended for these patients. 1. Preoperative evaluation and check consents 2. Place an IV. A 20 gauge is usually sufficient for a D&C, and at least an 18 gauge is required for a D&E. 3. Bicitra 30 cc PO, cold or Pepcid 20 mg IVP and Reglan 10 mg 4. Anesthesia: General Anesthesia Mask Apply monitors, dose with 2 mg versed in advance, fentanyl 20 to 100 ug, and titrate Propofol to deep sedation. Spinal Anesthesia (saddle block, sit for 3 to 5 minutes) 1.5% hyperbaric lidocaine - 45 mg (3 cc) to 60 mg (4 cc) Fentanyl - 10 µg Anesthesia for Postpartum Tubal Ligation Anesthesia for postpartum tubal ligation (PPBTL) is a common part of our practice. Typically these are done immediately after a vaginal delivery. Ensure that NPO status of the patient is appropriate. Other times, a PPBTL will be done at some later time designated by the obstetrician. In reference to the Practice Guidelines for Obstetrical Anesthesia “epidural, spinal, and general anesthesia can be provided without affecting maternal complications.” In addition “a postpartum tubal ligation can be performed safely within eight hours of delivery in many patients.” Remember women are at increased risk for aspiration at least up to 2 weeks postpartum. A PPBTL is an elective procedure that can be done at a later date as an outpatient laparoscopic tubal ligation. If it is clearly beneficial for patient safety to re-schedule a PPBTL, explain this to the obstetrician as early as possible. This does not occur often. Only 78% of labor epidurals used for PPBTL's, only hours after delivery, will work (25% fail to work adequately for surgical anesthesia). There is also a significantly longer time from anesthesia start to incision with epidural reactivation versus spinal anesthesia (J. of Clinical Anesthesia 7:380, 1995). If more than 4 hours have elapsed after delivery it is usually better to pull the epidural catheter and do a spinal anesthetic. Preparation for Epidural for PPBTL: 1. Check the consent 2. Co-Load: Each patient should have a working IV in place, and concurrent administration of Lactated Ringers's solution is recommended during epidural anesthesia. 3. Bicitra 30 cc PO, cold or Pepcid 20 mg IVP and Reglan 10 mg 4. Standard intraoperative monitoring 15 5. An epidural epinephrine test dose (15 µg) is effective in these patients. They are no longer laboring patients. Do not forget all the other signs of intravascular toxicity. 6. Local Anesthetics: 3% 2-chloroprocaine 15 to 20 cc of 3% 2-chloroprocaine 2 cc of sodium bicarbonate (a good choice for a typically short procedure) 2% lidocaine 15 to 20 cc of 2% lidocaine 2 cc of sodium bicarbonate 1 cc of fentanyl Preparation for Spinal for PPBTL: A spinal is a good choice for women without a pre-existing labor epidural in place and in women that do have a pre-existing epidural in place that has not been used for several hours. A labor epidural in a postpartum woman can become displaced after only a few hours and consequently not effective for operative anesthesia. An absolute duration for which an epidural can be considered "good" for operative anesthesia requires the judgment of the anesthesiologist. If several hours have elapsed since delivery and there is no evidence of epidural block present, removing the epidural catheter and administering a spinal anesthetic offers a reliable safe anesthetic for PPBTL. A recent article (Anesth Analg 2001; 93:1006) found that a small dose of intrathecal duramorph (100 µg) was significantly effective in relieving the postoperative pain associated with postpartum tubal ligation. This was a prospective placebo controlled study with a cohort of 66 women. Do not remove an epidural catheter from a patient and administer a spinal anesthetic if there is any evidence of a block present. A high spinal can result. This is well reported in the literature. 1. Check the consent 2. Co-Load: Each patient should have a working IV in place, and concurrent administration of Lactated Ringers's solution is recommended during spinal anesthesia. 3. Bicitra 30 cc PO, cold or Pepcid 20 mg IVP and Reglan 10 mg 4. Standard intraoperative monitoring 5. Spinal Solution: PPBTL Spinal Solution 1.5% hyperbaric lidocaine - 60 mg (4 cc) Fentanyl - 10 µg (0.2 cc) Duramorph 100 ug (0.2 cc of 5 mg / 10 ml) duration - 45 minutes Preparation for General Anesthesia for PPBTL: General anesthesia is an acceptable alternative as an anesthetic for PPBTL. Be careful to follow strict NPO guidelines as well as aspiration prophylaxis. Women in the postpartum period have demonstrated delayed gastric emptying of solid food when compared to a non-pregnant female population (Anesth Analg 1997; 84:522). Be confident that you can successfully control the maternal airway. Remember, this is an elective procedure that can be rescheduled when maternal anesthetic risk factors are minimized. Note – Although neuraxial techniques are preferred, GA with RSI can be performed the next day after delivery for an elective PPBTL, without waiting for over 2 weeks, as long as the patient has been NPO overnight, in cases such as patient refusal, patient preference for GA, etc., as long as the above precautions are followed (2007 Practice Guidelines for Obstetric Anesthesia). 16 Anesthesia for Cerclage Procedure Anesthesia for cerclage repair is typically for the McDonald technique. This technique involves a suture tightened around the cervical canal to reduce the diameter of the canal through a vaginal approach. The Shirodkar cerclage involves a transabdominal approach and will not be discussed below. Women that have a previous history of an incompetent cervix or women that have and active prolapse of membranes are candidates for cerclage. The obstetrician should document a viable fetus prior to the procedure. Cerclage is usually done in the second trimester and only rarely in the third trimester. A woman that has prolapse of her membranes may be kept in the Trendelenberg position for days prior to the procedure. Be aware that an extended period of time in the Trendelenberg position can cause venous congestion of the maternal airway resulting in a small glottic opening. Airway management can be hazardous in these patients. Although Lidocaine, both isobaric and hyperbaric, has been used spinally for the Cerclage procedure for many years, the risk of transient neurologic symptoms (TNS) has been a concern. Some studies have shown that TNS can occur even at lower concentrations of spinal lidocaine1. Newer studies, however, have shown TNS to occur with multiple local anesthetics, and that spinal lidocaine can still be a safe option, especially isobaric lidocaine2. In this section, we will explore some of the options for spinal for the Cerclage procedure. The importance of trendelenberg positioning in the parturient with cervical incompetence, especially in severe cases, with so-called “hourglassing membranes,” must be emphasized. An isobaric spinal can be placed in the setting of trendelenberg positioning, but a slightly hypobaric agent would be even more ideal in this setting resulting in a saddle block distribution of anesthesia. We can use a very interesting physical property of local anesthetic solutions which was studied in 1993 to aid us in finding this agent – a relationship between the density, specific gravity and baricity of spinal anesthetic solutions at body temperature. Agents which are isobaric at room temperature were found to become slightly hypobaric at body temperature3! We can use that to advantage here – give the agent in the setting of trendelenberg positioning, and as the solution is warmed to body temperature after it is injected, it will become slightly hypobaric and rise to produce a saddle distribution! Only one question remains unanswered now – which agent? Isobaric spinal lidocaine 60 mg plus fentanyl 10 mcg, is still a safe and viable option with a long track record. We have already seen that isobaric spinal lidocaine is safer than the hyperbaric – and we have also already seen that an isobaric solution is preferred in these cases. Spinal bupivacaine could be used, but ropivacaine may be better, as it is associated with less motor block, a good characteristic for a short case such as a cerclage. Using ropivacaine spinally is offlabel, but it is very acceptable. It can and often is used in combined spinal epidurals as well, for the same reason – less motor block4. Fentanyl can be added as well, for its’ synergistic effects. How much? Again, the literature can guide us – 10 to 15 micrograms has shown to produce the best side effect to benefit ratio5. The optimal doses of ropivacaine and bupivacaine in combination with fentanyl have also been studied. Ropivacaine 10-20 mg with fentanyl 10- 15 mcg is acceptable, as is bupivacaine 5-10 mg with fentanyl 10-15 mcg. Preparation for Cerclage Anesthesia: 1. Check the preoperative status, consents, NPO status, and type of cerclage (i.e. is it elective or a high risk urgent procedure) 2. 18 gauge IV 3. Hydrate with lactated Ringer's solution 1000 to 1500 cc 4. Bicitra 30 cc po 5. Standard intraoperative monitors 6. If the cerclage is a high risk urgent procedure with prolapse of membranes be prepared to pharmacologically relax the uterus (nitroglycerin, magnesium sulfate or terbutaline). 7. Fentanyl IVP for sedation 8. Spinal Anesthetic: Isobaric 2% plain lidocaine is an excellent choice because the spinal can be placed with the patient in the Trendelenberg position. If a patient that does not begin the procedure in the Trendelenberg position the obstetrician typically will ask for steep Trendelenberg to optimize surgical exposure. Isobaric 2% plain lidocaine usually behaves mildly hypobaric which is ideal for the procedure. Cerclage Spinal Solution 2% lidocaine plain (isobaric) 60 mg (3 cc) 0.5% plain bupivacaine (isobaric) 5 to 10 mg Fentanyl 10 µg 17 Nitrogylcerin Use In The Pregnant Patient NTG administered IV will produce uterine relaxation with a minimal decrease in maternal blood pressure. NTG may be used in place of a volatile anesthetic with endotracheal anesthesia when uterine relaxation is desired. Be prepared to use NTG when the obstetrician asks for uterine relaxation. The following are indications for the use of NTG in the pregnant patient: 1. Retained placenta: There are no prospective studies that demonstrate a clear benefit of NTG in this situation. If NTG is chosen, check maternal blood loss and hemodynamics, and administer prior to and without the concomitant use of pitocin. My experience has not been very successful with NTG for retained placenta. Several doses may be required (see below). 2. Twin, Breech or Complicated Delivery: Delivery of the fetal head during a breech delivery (cesarean or vaginal) or the delivery of a second twin often requires uterine relaxation. Uterine relaxation during these deliveries, when required, may be life saving for the fetus and decrease maternal morbidity by preventing extension of a surgical incision or cesarean section. 3. Cerclage: An unpublished use for NTG is in the patient for a high-risk cerclage. These patients have prolapsed membranes that have a high risk of rupture during the cerclage procedure. The uterine relaxation provided by NTG helps to reduce the amount of membrane prolapse. This makes the procedure much easier for the obstetrician. I have found these patients, that are earlier in gestation, require an increased dose of NTG. An IV bolus of between 200 µg and 400 µg has been effective. Incremental doses of ephedrine 5 to 10 mg have also been required to maintain optimal maternal-fetal perfusion. (Please refer to the section on Cerclage in these Guidelines for more information about this procedure). 4. Interventional Tocolysis for fetal distress (Presented in a separate section in these Guidelines). Nitroglycerin Dose 50 to 100-µg IVP q 1-2 minutes, onset within 30 seconds Dose NTG for the desired clinical effect as maternal hemodynamics tolerate “Wet Taps” "WET TAPS" and postdural puncture headaches need to be given serious attention. Chadwick et al (Anesthesiology 74:242, 1991) showed that this was one of the most frequent closed claims settled against anesthesiologists that practice obstetric anesthesia. The cost of settlement can approach $20,000. An excellent prospective study (Anesthesiology 2001; 95:334) of 504 patients with a postdural puncture headache that received an EBP revealed: 1) 75% had complete relief, 18% had incomplete relief, and 7% no relief after a single EBP 2) The 7% failure rate was associated with larger needle size and a “decreasing delay between dural puncture and EBP” 3) The 19 women that had a second EBP 2 remained failures. This is excellent information to tell our patients that develop a postdural puncture headache. When an obstetric patient is "wet" tapped: 18 1. Write in large letters on the anesthesia record WET TAP. Clear documentation helps the person called to see the patient complaining of a headache. 2. Post the patient name and room number in the anesthesia office on labor and delivery. This is to insure that the patient is rounded on the following day. 3. This patient should then be rounded on each day she is in the hospital inquiring on her neurologic status. A note should be made in the chart to this effect. "Patient ambulating without symptoms, will follow-up." OR "Patient with a postdural headache, nausea and photophobia, will perform an epidural blood patch." 4. When a patient is diagnosed with a postdural puncture headache (PDPH) and an epidural blood patch (EBP) is done, she should be rounded on the following day to insure a successful result from the EBP, or called at home. If a patient is being treated conservatively she needs to be contacted regularly until the PDPH is resolved. 5. If a patient develops a PDPH at home, she should be called at home until the PDPH is resolved spontaneously (if an EBP is declined) or brought to the hospital for an EBP. An option for patients that are “wet tapped” when a labor epidural is attempted is an intrathecal catheter. When the “wet tap” is recognized, thread the epidural catheter 3 cm into the subarachnoid space. Clearly label the catheter “Intrathecal” with a red medication sticker. Dose the intrathecal catheter with 1cc (2.5 mg) of 0.25% bupivacaine and 25 ug of fentanyl. Begin a continuous infusion of our standard epidural solution at 2 cc per hour or set a PCEA with a basal rate of 1 cc/hr and 1 cc with a lockout of 30 minutes. If air is injected intrathecally, the patient will complain of a headache immediately when she sits up (pneumoencephalus). The air will rise and irritate the cerebral ventricles, but will ultimately be absorbed. If the decision is made to simply go to another interspace and place an epidural catheter, don’t forget to use saline loss of resistance and not air. The air can find its way across the dural rent and cause an immediate maternal headache. Intrathecal Catheter after a “WET TAP” Initial Dose: 1cc (2.5 mg) of 0.25% bupivacaine and 10 ug of fentanyl Continuous Infusion 2 cc/hr Or PCEA 1 – 2 cc/hr and 1 cc with a lockout of 30 minutes (Use the standard epidural solution) Considerations: It is very important to inform the patient and all involved staff of the unintentional dural puncture, and to document it in the chart. This way, the patient can receive proper follow-up. Not all headaches after epidurals are PDPH! Especially beware of meningitis, as it is unbelievably lethal – patients can succumb after a couple of days from it! Both meningitis and PDPH can present with nuchal rigidity, photophobia, and nausea and vomiting. Fever can be a late sign in meningitis, and can be a nonspecific finding in the parturient anyway. The key clinical feature of PDPH is that it is a positional headache –it worsens with sitting up and is reduced in intensity by laying down. As a side note, as far as meningitis is concerned, prevention is always better than cure. The importance of strict aseptic technique while performing spinals, epidurals or blood patches cannot be overemphasized. Although bed rest was once recommended for PDPH, it is no longer encouraged, as it has been shown to merely delay the onset of PDPH, and, in addition, increases the risk of DVT/PE. The parturient is already at high risk for PE, and the last thing that should be recommended for them is bed rest! The use of prophylactic blood patch was also popular in the recent past. This too, however, has been shown to merely delay the onset of PDPH1. Additionally, it is not an entirely benign procedure – it carries a risk of infection, and may even make future epidurals less effective by causing adhesions in the epidural space. Furthermore, 35% of patients who receive a blood patch report back pain, and a few have even developed radiculitis. Finally, it is not uncommon to obtain a second tap while attempting an EBP! Epidural saline injections through the catheter (30-60 ml of sterile NS through the epidural catheter q 6 hours X 4 doses) may be done instead, as it is relatively benign, but keep in mind that this too will most likely merely delay the onset of PDPH, and is therefore not routinely recommended. Conservative measures should always be recommended to the patient first, but the risks and benefits of all treatment modalities should be explained. The cornerstone of conservative therapy is hydration. Encourage the patient to increase PO intake of fluids. Caffeine, both IV and PO, has been shown to be beneficial in relieving PDPH2 -- the mechanisms involved include cerebral vasoconstriction and increased CSF production in the Choroid Plexus. Encourage the patient to drink caffeinated beverages such as coffee, and explain to them that this can be helpful for the headache. Make sure that the patients’ overall fluid intake is increased, as otherwise the diuretic effect of the caffeine may dehydrate them. This has not been shown to be a major concern, however. Intravenous caffeine has been shown to be better than PO caffeine alone, and patients can safely receive it even while they are consuming caffeinated beverages. It is given in the form of Caffeine Sodium Benzoate, 500 mg in 1 liter of IV fluid, to be infused over 1 hour – repeat q 8 hours. Avoid in preeclampsia or known seizure disorder3. Additionally, Zofran 4 mg IV q 6 hours prn may be given for nausea. Ketorolac 30 mg IV q 6 hours, or, alternatively, Naproxen 550 mg po q 12 hours can be helpful as well. No NSAID has been shown to be more effective than any other for PDPH. Percocet PO q 4 hours can be added for more severe pain. Although there is some evidence that spinal catheters (threading the epidural catheter through the dural rent) can help prevent 19 PDPH, consider avoiding them. Accidental mismanagement of them can be lethal – for example, a fatigued clinician giving an epidural-strength C-Section dose of local anesthetic into a spinal catheter would have disastrous consequences! Epidural blood patch has been considered the gold standard for PDPH treatment -- but, as mentioned above, should not be offered prophylactically. Even if conservative measures merely delay the onset of PDPH, they can reduce its’ severity, and can be adequate. Furthermore, EBP has been shown to be more effective if performed more than 12 to 24 hours after the dural puncture, and this should be explained to the patient as well4. The safest strategy is to offer conservative treatment first, then perform the blood patch only if those treatments fail, and then only after at least 12 hours have passed since the tap. Risks versus benefits should be explained. Strict aseptic technique must be followed, and the patient should lie supine, as flat as possible, for at least 30 min after the procedure to improve its’ effectiveness. Coagulation Status and Regional Anesthesia An epidural hematoma is a rare but potentially catastrophic event. Evaluation of a patient’s coagulation status before placement of a spinal or epidural and before removal of an epidural catheter is mandatory when an abnormality in coagulation is suspected. A spinal should not be considered any less of a risk than an epidural as a risk factor for epidural hematoma. The ranges of laboratory values that allow the safe administration of regional anesthesia do not exist. It is always a risk benefit ratio driven by clinical assessment and judgment of the benefits of regional anesthesia versus the risk of epidural hematoma. Always document your thought process. If you choose a regional anesthetic in a patient with abnormal lab values (i.e. a preeclamptic with a difficult airway that is given a regional block for C/S) it is a good idea to document intact lower extremity function until coagulation status returns to normal. Typically I will order q 2 documentation of lower extremity function by the nurse. Assessment Guidelines: The following are conservative guidelines for assessing coagulation status in obstetric patients. Remember this is always a risk benefit decision made in the best interest of the patient. platelet count - > 100,000/ µm3 PT, PTT - < 1.5 times normal fibrinogen - > 100 mg/dL Clinical correlation: Check lab values early so when a patient is a candidate for regional anesthesia you are ready and they do not have to wait for pain relief. Mild Preeclampsia If a screening platelet count is > 150,000 the risk of an abnormal PT or PTT is < 2% Severe Preeclampsia Check platelet count, PT and PTT Recent studies (Anesth Analg 1997; 85:385, Anesthesiology 1999; 90;385, IJOA 2001; 10:113) indicate that with a proper maternal history and physical that a regional anesthesia can be conducted with a platelet count > 75,000 mm-3. HELLP Syndrome: Considered a form of severe pre-eclampsia, HELLP syndrome is also a clinical entity in and of itself. It is found in 0.2 to 0.6% of all pregnancies, and in 10 to 20% of pre-eclamptic patients. HELLP syndrome has some rather unusual features in that although the hypertension and proteinuria seen in preeclampsia can be quite mild in HELLP syndrome, severe hematological abnormalities can be seen there. Microangiopathic hemolysis can be severe enough to cause anemia, and DIC complicates 20% of cases (85% of cases involving acute renal failure). Nevertheless, HELLP syndrome is not necessarily a contraindication to neuraxial anesthesia – many cases have safely and successfully employed neuraxial techniques (Wong, Cynthia, from the textbook: Spinal and Epidural Anesthesia). As long as a platelet count is over 75,000, the trend in plt count is good, and other coagulation parameters are normal, it is safe as long as the plt count is over 75,000 before catheter removal, as well. Other useful labs include CBC, chem-7, LFT’s, LDH (elevated by hemolysis), and renal function. Fibrin degradation products and d-dimer, markers of subclinical DIC, have been used to help predict which patients with preeclampsia are at higher risk of developing the HELLP syndrome. (Padden, MO, HELLP Syndrome, recognition and perinatal management, American Family Physician, 60(3):829-36, 839). 20 Very little research has been done on what constitutes a safe level of platelets to proceed with neuraxial anesthesia, and there is much controversy on what the lowest safe limit of platelet count actually is. In fact, it must be emphasized again that there are no set numbers, only rough guidelines – to be taken in the context of the patients’ entire clinical picture. A lower platelet count may be accepted in a parturient with a suspected difficult airway, for example. One thing that is widely agreed, however, is that it is not necessary to obtain routine platelet counts on healthy parturients. It is only necessary to check a screening platelet count on patients with pregnancy-related hypertensive disorders, such as pre-eclampsia, HELLP syndrome, etc1. The rough guidelines that have been published tend to indicate that greater than 100,000 is clearly safe, but the risk is acceptable at a level above 75,000 as well. Some even say that greater than 50,000 could be acceptable in certain cases, such as ITP, where platelets may be giant, clumped, but functional2. In the normal, healthy parturient, platelet counts can vary over time -- and even transiently dip below 75,000, but this is not necessarily cause to abandon neuraxial anesthesia. What is really important is where the trend is heading. If the patient is clinically healthy, with no clinical evidence of coagulopathy or pregnancy-related hypertensive disorders, the platelets should be functional as well. Again, there is no need to even check or monitor the platelet count in a healthy parturient in the first place! A good history and physical can rule out many coagulopathies. Bleeding time has not been shown to be a helpful parameter in assessing patients for coagulopathy, as it often doesn’t correlate well with clinical bleeding. 21 Interventional Tocolysis for Fetal Distress: Interventional tocolysis is defined as maternal administration of a pharmacologic agent to decrease uterine muscular activity allowing increased intervillous blood flow. The goal of intrauterine resuscitation is to increase intervillous blood flow by optimization of maternal factors. Preparation for Interventional Tocolysis: 1. Correct the maternal position. Left uterine displacement allows maximum uterine blood flow. 2. Correct maternal hypotension with an intravenous fluid bolus and intravenous ephedrine. 3. Administer supplemental maternal oxygen. 4. Discontinue the oxytocin infusion. 5. Administer intravenous 0.25 mg of terbutaline. Considerations: The sequence of the above algorithm should be changed to fit the clinical situation. Intravenous administration of 0.25 mg of terbutaline is an effective treatment for fetal distress (Am J Obstet Gynecol 1989; 160:615) resulting in an immediate decrease in uterine activity. Subcutaneous treatment with terbutaline will result in a delay of uterine response for a few minutes. Terbutaline is not appropriate in parturients with severe abruptio placentae, maternal cardiovascular disease or other conditions worsened by betaadrenergic stimulation. If fetal recovery does not improve after all methods of intrauterine resuscitation have been exhausted, the neonatal condition is typically improved after operative delivery. When evaluating a parturient with intrauterine fetal distress, it is very important to consider the cause of the fetal distress. Maternal supine position, recent paracervical block, or uterine hyperstimulation from oxytocin infusion can often be successfully treated by following intrauterine resuscitation measures without tocolysis1. These measures include correcting the maternal position – left or right uterine displacement, correcting maternal hypotension with an IV fluid bolus and IV ephedrine, administering supplemental maternal oxygen, and discontinuing the oxytocin infusion. If Interventional tocolysis is required, however, terbutaline is no longer the only intravenous pharmacologic agent in our armamentarium. Recent studies have supported the use of IV nitroglycerin as well, for acute intrapartum fetal resuscitation. It was shown that although terbutaline could provide effective tocolysis with less impact on maternal systemic blood pressure, no difference between the drugs was noted in terms of the success of acute intrapartum fetal resuscitation2. In some cases, nitroglycerin may be a better choice than terbutaline – for example, in cases where terbutaline is best avoided, such as severe abruptio placentae, maternal cardiovascular disease, uncontrolled maternal hypertension, or conditions worsened by beta– adrenergic stimulation, as was mentioned previously. Both agents have the advantage of a rapid onset of action. PCA for Labor Analgesia In the rare instance that an epidural is not used for labor analgesia there is a good option for IV PCA. Initial investigation with remifentanil (Anesth Analg 2000; 91:606) using varying doses and lockout periods did not show satisfactory results. A subsequent study (Anesth Analg 2002;94:913) found that remifentanil was associated with “maternal oxygen desaturation, sedation, and reduced fetal heart rate beat-to-beat variability.” In addition there was wide individual variation in the dose required for effective labor analgesia. Ketamine is a known powerful analgesic with pharmacologic properties that block NMDA receptors. This acts to potentiate opiates. A combination of low dose ketamine and fentanyl works well as a PCA mixture for laboring patients. Mixture: Ketamine 0.5 mg/ml Fentanyl 2.5 ug/ml PCA Settings: Basal Rate 10 cc/hr Bolus 10 cc q 15 min 22 CPR for the Parturient: Cardiac arrest in the laboring patient is an infrequent occurrence. As such it requires an understanding on how to proceed if a patient codes during labor. Perimortem cesarean section is recommended within 4 minutes of cardiac arrest in pregnant women beyond 24 weeks gestation as a resuscitative measure for mother and neonate if the resuscitative measures are not immediately successful (Obstet Gynecol 1986; 68:571 and ACLS). Please consider the following algorithm for resuscitation of the pregnant patient. Airway: ■ ■ Don’t forget that intubation is more difficult in the pregnant patient Capillary engorgement of the respiratory tract mucosa can result in excessive bleeding when the nose is instrumented Breathing: ■ The pregnant patient requires higher minute ventilation. Pregnant patients have a resting pCO2 of 32 torr and a decreased HCO3-. Subsequently the pregnant becomes significantly more acidotic during the hypoxia accompanied by cardiac arrest. Circulation: ■ Left uterine displacement (a roll under the right hip) to offset aortcaval compression is essential immediately for effective chest compression. Delivery, Drugs and Defibrillation: ■ ■ ■ Perimortem cesarean section is recommended within 4 minutes of cardiac arrest in pregnant women beyond 24 weeks gestation as a resuscitative measure relieves aortocaval compression and improves circulation. Down regulation of α and β receptors (up regulation in preeclamptic patients) increased intravascular volume and decreased protein binding should be considered for dosing. Adenosine can be used without adverse fetal effect. Treat the mother and consider the fetus secondarily. Defibrillation as in non-pregnant patients. The fetus is typically protected from maternal defibrillation. Postpartum Hemorrage Postpartum hemorrhage remains a major cause of maternal arrest and resuscitation efforts. Karpati (Anesth 2004; 100:30) found that 51% of the 55 paturients studied had elevated serum levels of cardiac troponin I associated with EKG sign of ischemia and deteriorated myocardial contractility. The severity of the hemorrhagic shock correlated with the degree of myocardial contractility. These results suggest that the treatment of postpartum hemorrhage-induced shock should be coupled with concomitant prevention of myocardial ischemia even in young patients. Postpartum Hemorrhage Continued -- Placenta Previa and Placenta Accreta: The incidence of placenta accreta has increased 10-fold over the past 50 years, and women with 2 or more cesarean deliveries are at a 45% increased risk for it (ACOG Committee opinion 266, 2002). It, along with placenta previa, is a major cause of postpartum hemorrhage and gravid hysterectomy. The risk of developing placenta previa is increased 8x after 4 or more C-sections (Gilliam, Obstet Gynecol 2002; 99:976). Other risk factors for both placenta previa and placenta accreta include advanced maternal age, prior suction D and C, prior myomectomy, cigarette smoking and multiparity. Placenta previa and placenta accreta may occur together. Placenta accreta is further classified into placenta increta and placenta percreta, depending on the depth of invasion of the placenta. “anesthesiologists should be prepared for major hemorrhage in all cases of placenta accreta” (Weiniger CF, Anesth 2005; 60:1079). Fr both placenta previa and placenta accreta, large bore IV access, and the rapid availability of type and matched blood is imperative. Central venous access and arterial pressure monitoring should be strongly considered. Additionally, embolization catheters can be placed pre-operatively in the hypogastric arteries in the interventional radiology suite. The embolization balloons can be inflated intraoperatively if necessary. Both previa and accreta can be diagnosed by ultrasound, and MRI can confirm placenta accreta in difficult cases. Intraplacental lacunae are seen on ultrasound in placenta accreta (Mazouni, Placenta, 2007; 28:599). The main treatment for placenta accreta is, unfortunately, gravid hysterectomy (Mazouni, Placenta, 2007; 28:599). Neuraxial anesthesia (epidural or CSE) is an excellent choice for these patients, as long as help is available, and facilities for the rapid induction of GA and the rapid infusion of blood and blood products are available. The epidural can provide excellent postop pain management as well. Check coagulation profiles before removing the catheter. It is also well-supported and acceptable to start the case under neuraxial anesthesia for the urological procedures sometimes performed (such as ureteral stenting to protect the ureters 23 during hysterectomy), and the delivery of the fetus, and then later convert to GA if the surgical team encounters a high potential for uncontrolled hemorrhage. Be ready to convert to GA at any point in these cases. If a massive hemorrhage or abruption occurs pre-delivery, the anesthesiology team should be ready to perform a crash induction/general anesthesia if necessary – in such cases, there may not be enough time to perform neuraxial anesthesia -- and even if an epidural catheter is already in situ, if the patient becomes obtunded from massive hemorrhage, the airway must be intubated anyway. As a side note, anytime there is massive hemorrhage or there is grave danger to the fetus as indicated by “loss of fetal heart tones”, etc., the fastest way to facilitate delivery in those cases is usually crash general anesthesia. It is vital that the obstetrician communicates the proper urgency of the delivery to the anesthesiology team. A multidisciplinary approach is vital, and clear lines of communication between all members of the clinical team must remain open at all times. Both Placenta previa and placenta accreta are becoming more common, and demand the utmost of caution. Fetal Demise Emotionally devastating, this is a very difficult thing to deal with for all parties -- the parturient, her family and all caregivers. Sometimes we are called to provide epidural analgesia to these patients. Treating the patient and her family with compassion and dignity is essential in these cases. Additionally, it is very important not to point any blame on anyone or any clinician for the fetal demise. Epidural analgesia can be very beneficial in these cases, as easing the physical suffering helps the patient and her family focus on and work through their emotional distress. From a clinical standpoint, one of the biggest concerns in these cases is the potential for coagulopathy – in the past, some have even declined providing epidural analgesia for these patients for that reason. The biggest risk of coagulopathy is with gestational age over 14 weeks, and with a longer period of time of the dead fetus in utero. Studies have shown, however, that coagulopathy is not as prevalent in these cases as was previously thought. In fact, coagulopathy is rare in cases where the fetus and placenta are delivered within one week of fetal demise1. A coagulation profile should be obtained, and if normal, it is safe to proceed with the epidural, as long as an expeditious delivery is planned. Neuraxial Opioid-Induced Pruritis The Incidence of opioid-induced pruritis has been reported to vary between 30-100%, making it the most common side effects of neuraxial opioids. The mechanisms involved are not fully understood, but are not believed to be related only to histamine release, as antihistamines are sometimes not very effective in treating neuraxial opioid-induced pruritis. Recent studies have suggested that the mechanisms may involve central 5-HT3 receptors in the dorsal horn of the spinal cord, and the trigeminal nucleus of the medulla. These same studies have shown a beneficial effect of 5-HT3 blockers in standard doses, such as Zofran 4 mg IV, in the treatment of this pruritis1. Some have used naloxone to treat the pruritis – this can nullify the analgesia as well, even at low doses. Some studies have shown that nalbuphine, a mixed agonist/antagonist, provides better relief than low-dose naloxone --with less antagonism of analgesia1. Intralipid for Bupivacaine Toxicity Bupivacaine exhibits severe cardiotoxicity at high doses, and can lead to rapid cardiovascular collapse with refractory cardiac arrest. In the past, treatments were few, and included prolonged CPR and even cardio-pulmonary bypass for prolonged periods. Intralipid 20%, a lipid emulsion, is a relatively new treatment for this very lethal condition. If signs of bupivacaine toxicity occur (cardiovascular collapse, loss of consciousness with or without seizure, etc.) -- stop the injection, call for help, secure the airway, and administer 100% oxygen. Confirm intravenous access. Initiate CPR. Treat seizures with small incremental doses of a benzodiazepine, thiopental or propofol. Consider cardiopulmonary bypass if available. Begin Intralipid therapy at the same time as these other interventions, and continue CPR as it is being administered – effective compressions are obviously required to circulate the agent. Start with an intravenous bolus dose of Intralipid 20%, 1.5 ml/kg, then begin a continuous infusion of 0.25 ml/kg/min for 30 min. This can be repeated 1-2 times if no clinical improvement is seen. Note – the infusion can be increased to 0.5 ml/kg/min for 60 min if the cardiovascular instability worsens at any point. 24 The most important thing to realize in these cases is that although Intralipid can hasten recovery from bupivacaine toxicity, one must still be very patient in the resuscitation efforts of these patients. Very prolonged CPR may be required. Although Intralipid is generally recommended to be given after cardiac arrest has occurred, it has been given in the setting of cardiovascular collapse before arrest in bupivacaine toxicity with good success1. Two mechanisms by which Intralipid works have been suggested. The first is that the Intralipid acts as a “lipid sink” into which the lipophilic bupivacaine binds and partitions into. The second is that the lipid itself provides fatty acid substrate for myocardial cells to metabolize and produce ATP at the mitochondrial level. 25 References References for Aseptic Technique for Neurazial Anesthesia: 1. 2. 3. 4. Three Cases of Bacterial meningitis After Spinal and Epidural Anesthesia, M. Trautmann, European Journal of Clinical Microbiology and Infectious Diseases, Volume 21, Number 1 / Jan. 2002. Hughes, E.W., a Staph aureus paraspinal abscess associated with epidural analgesia in labor. Anaesthesia, 2001. Safe Injection Practices to Prevent Transmission of of Infections to Patients, Excerpted from the CDC Guidelines for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings 2007. Hospital Policy for Prevention of Infection after Neuraxial Blocks in Obstetrics, B. Benhamou, International Journal of Obstetric Anesthesia, Volume 11, Issue 4, Pages 265-269. References for Combined Spinal-Epidural: 1. 2. 3. 4. P. Pan, T. Bogard, M. Owen. Incidence and characteristics of failures in Obstetric neuraxial analgesia and Anesthesia: a retrospective analysis of 19,259 deliveries. Int. Journal of Obstetric Anesthesia, Volume 13, Issue 4, pages 227-233. ASA Guidelines, 2007. C. Wong, Efficacy and side-effect profile of varying doses of intrathecal fentanyl added to bupivacaine forlabor analgesia. Int. Journal of Obstetric Anesthesia, Volume 13, Issue 1, pages 19-24. D.A. McNamee, Spinal anesthesia: comparison of plain ropivacaine with bupivacaine for major orthopedic Surgery. British Journal of Anaesthesia, 2002, vol 89, no. 5 702-706 References for Cerclage chapter: 1. 2. 3. 4. 5. 6. Luiz, EI, Lidocaine 2% with or without glucose 8% for spinal anesthesia for short orthopedic surgery. Can. J of Anesthesia 52:887-888(2005). F Hampl, MC Schneider, H Pargger, J Gut, J Drewe and K Drasner, (Dept. of Anest. Univ of Basel/Kantonsspital, Switzerland) A Similar incidence of Transient neurologic symptoms after spinal anesthesia with 2% and 5% lidocaine, Anesthesia and Analgesia, Vol 83, 1051-1054, 1996. Terese T. Horlocker and Denise J. Wedel, (Dept. of Anest. Mayo Clinic, Rochester, Minnesota) Density, specific gravity and baricity of spinal anesthetic solutions at body temperature, Anesthesia and Analgesia, 1993; 76: 1015-1018. D.A. McNamee, Spinal anesthesia: comparison of plain ropivacaine with bupivacaine for major orthopedic surgery, British Journal of Anaesthesia, 2002, vol 89, no. 5 702-706. C. Wong, Efficacy and side effect profile of varying doses of intrathecal fentanyl added to Bupivacaine for labor analgesia. Int. Journal of Obstetric Anesthesia, Volume 13, Issue 1, pages 19-24. Yaakov, B., Subarachnoid small-dose bupivacaine versus lidocaine for cervical cerclage. Anesthesia and Analgesia, 2003; 97:56-61. References for Wet Tap chapter: 1. 2. 3. 4. Vasdev GM, Southern PA: PDPH. The role of prophylactic EBP. Curr Pain Headache Rep 2001; 5: 281-83 Schzer PH, Abel H. Post spinal anesthesia headache treated with caffeine, Evaluation with demand method. Part 1. Curr Therp Res 1978; 24: 307-12. Kotur, P.F. Evidence Based Management of PDPH, Indian J. Anaesth. 2006; 50(4) : 307-308 Safa TV, Thormann F et al. Effectiveness of EBP in the management of PDPH, Anesthesiology 2001; 95: 334-39. References for Platelet Count and Neuraxial Anesthsia chapter: 1. 2. Practical Guidelines for OB Anesthesia, Oct. 18, 2006. 2 Sanjay Datta, Anesthetic and Obstetric Management Of high risk pregnancy, 2004. (textbook). References for Interventional Tocolysis for Fetal Distress chapter: 1. 2. 26 Dr. Frank Rosinia, M.D., author of Chapter 6: Uteroplacental Circulation, Interventional Tocolysis for Fetal Distress, of the textbook: Common Problems in Obstetric Anesthesia, Second Edition, Sanjay Datta, M.D. Pullen KM, Riley ET. Randomized comparison of intravenous terbutaline vs. nitroglycerin for acute intrapartum fetal resuscitation. Am J Obstet Gynecol. 2007 Oct; 197(4):414.e1-6.