Element Symbol Hyphen Notation Nuclear Symbol Protons (p+
advertisement
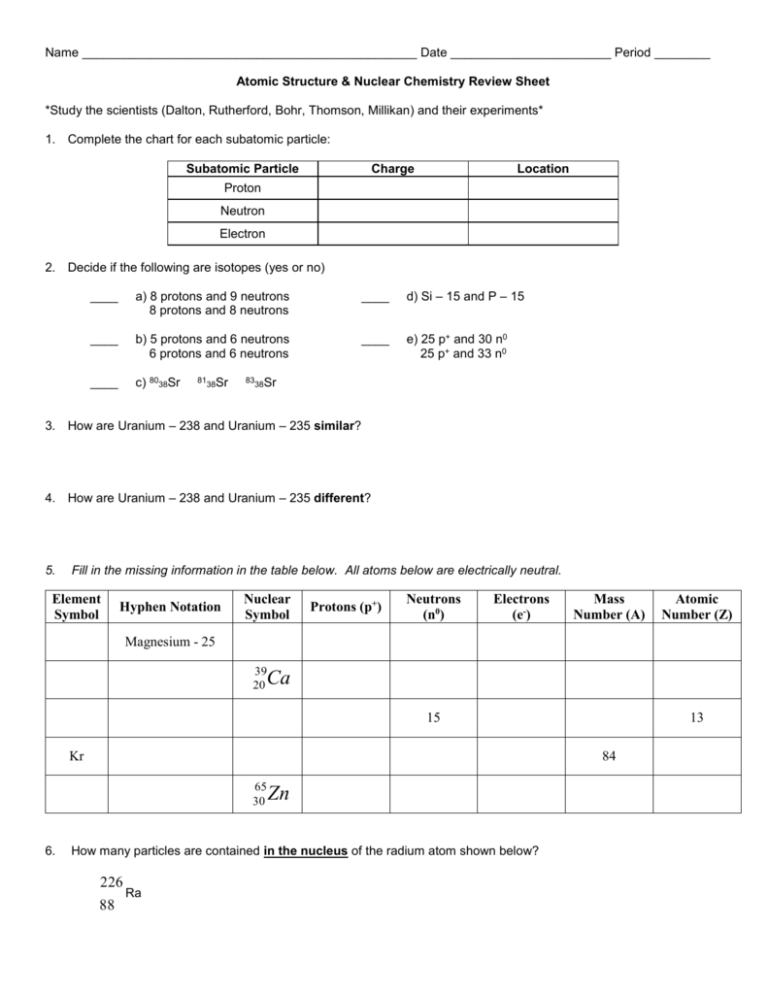
Name ________________________________________________ Date _______________________ Period ________ Atomic Structure & Nuclear Chemistry Review Sheet *Study the scientists (Dalton, Rutherford, Bohr, Thomson, Millikan) and their experiments* 1. Complete the chart for each subatomic particle: Subatomic Particle Charge Location Proton Neutron Electron 2. Decide if the following are isotopes (yes or no) ____ a) 8 protons and 9 neutrons 8 protons and 8 neutrons ____ d) Si – 15 and P – 15 ____ b) 5 protons and 6 neutrons 6 protons and 6 neutrons ____ e) 25 p+ and 30 n0 25 p+ and 33 n0 ____ c) 8038Sr 81 38Sr 83 Sr 38 3. How are Uranium – 238 and Uranium – 235 similar? 4. How are Uranium – 238 and Uranium – 235 different? 5. Fill in the missing information in the table below. All atoms below are electrically neutral. Element Symbol Hyphen Notation Nuclear Symbol Protons (p+) Neutrons (n0) Electrons (e-) Mass Number (A) Atomic Number (Z) Magnesium - 25 39 20 Ca 15 Kr 84 65 30 6. 13 Zn How many particles are contained in the nucleus of the radium atom shown below? 226 88 Ra For #s 7-9, calculate the average atomic mass or the % abundance in the problems. Show all equations and correct substitution of values with units. Round all answers to the hundredths place. 7. Naturally occurring element X exists in three isotopic forms: X-28 (27.977 amu, 92.21% abundance), X-29 (28.976 amu, 4.70% abundance) and X-30 (29.974 amu, 3.09% abundance). Calculate the average atomic mass #7 Answer: 8. Naturally occurring iron contains 5.82% iron-54, 91.66% iron-56, 2.19% iron-57 and 0.33% iron-58. Calculate the average atomic mass of iron. #8 Answer: 9. Gallium has two naturally occurring isotopes, Ga-69 and Ga-71. If the average atomic mass of gallium is 69.72 amu, what is the percent abundance of each isotope? #9 Answer: % ab. of Ga-69 = ____________ % ab. of Ga-71 = ____________ 10. Complete the chart: Type of Radiation/Particle Symbol Penetrating Power Material needed to stop/shield radiation Beta Alpha Gamma Proton Neutron 11. The diagram below represents the three types of radiation passing through an electric field. Which type of radiation (Alpha, Beta or Gamma) does each arrow represent? 12. Indicate the nuclear symbol of the missing reactant or product in each of the following nuclear reactions. a. 59Co 2H 0 e ______ 1 27 1 c. b. 22P 0e _______ 15 1 d. _______206Pb 4He 82 2 42Ti ______ 42 Sc 22 21 13. The following equation is a representation of what happens to the nucleus during beta radiation. What happens to the mass number? a. 1 n 1H 0e 0 1 1 _____________________________ 14. Given the equation: 6 C 7 N X? 14 14 What happens to the atomic number? __________________________________ X , what is the name and nuclear symbol of the particle is represented by the letter Write a balanced equation for each nuclear process in #s 15 and 16. 15. Beryllium-9 absorbs an alpha particle and emits a neutron. 16. Cobalt-59 absorbs a neutron and emits an alpha particle. 17. The decay series of uranium-235 is α, α, α, α, β, β, α. Write a balanced equation for each step in this decay series. Step 1: __________________________________________________ Step 2: __________________________________________________ Step 3: __________________________________________________ Step 4: __________________________________________________ Step 5: __________________________________________________ Step 6: __________________________________________________ Step 7: __________________________________________________ 18. If you have an original sample of 300 grams of polonium, how much polonium would be left after 2 half-lives? #18 Answer: 19. If you have an original sample of 50 grams of polonium and the half-life of polonium is 4000 years, how many years would it take to only have 3.125 grams of polonium left? #19 Answer: 20. Which type of reaction does the diagram to the right illustrate? a. b. c. d. Alpha Decay Fusion Fission Beta Decay 21. During a fission reaction, what type of particle is captured by a nucleus? a. b. c. d. Proton Neutron Electron Deuteron 22. Which equation represents a fusion reaction? 40 Ar 11H 19 K 01n a. 40 18 b. 234 91 1 Pa234 92 U 0 n c. 226 88 4 Ra 226 86 Rn 2 He d. 3 1 H 11H 24 He 23. What is the nuclear symbol for the missing product in the nuclear fission reaction? 1 U 01n139 56 Ba ______30 n 235 92






