A. Cellular Physiology a. Describe the cell membrane and its
advertisement

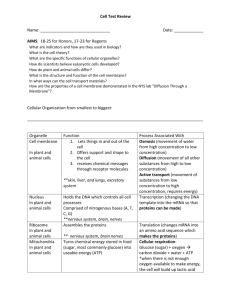
A. Cellular Physiology a. Describe the cell membrane and its properties. b. Describe the functions of mitochondria, endoplasmic reticulum and other organelles. Plasma membrane semipermeable lipid bilayer 7.5 nm thick phospholipids, cholesterol proteins structural pumps active ion/molecule transport channels receptors clathrins cluster to endocytose bound ligands insulin & other peptides, lipoproteins, viruses enzymes intercellular connections tight junctions desmosomes belt with bands of filaments containing actin spot central stratum of filaments hemi- epithelial cell to connective tissue gap junctions connexons allow molecules up to 800 d to pass rise in Ca2+ closes Cytoplasm Golgi complex vesicles → secretory granules Endoplasmic reticulum (rough and smooth) RNA → protein transcription in ribosomes glycosylation of proteins formation of vesicles and lysosomes Lipid droplets Lysosomes merge with (auto-)phagocytic vacuoles and release ribonuclease, deoxy~, phosphatase, glycosidases, arylsulfatases, collagenase, cathespins release of enzymes into the cell causes damage in vit A toxicity, ?gout enzyme defects cause lysosomal storage disorders Mitochondria separate DNA (female lineage) outer and inner membrane with cristae operate the citric acid cycle “cellular respiration” → ATP Secretory granules Centrioles 2 cylinders and right angles near nucleus made of 9x3 microtubules form the mitotic spindle in cell division Microfilaments long fibres of actin 4-6 nm diameter operate microvilli and attach to belt desmosomes Microtubules 25 nm diameter tubules made of α and ß tubulin (5 nm thick) maintain cell shape, constantly form and disassemble Cilia Cellular metabolism 1.A.1 James Mitchell (December 24, 2003) contain 9x2 +2 microtubules and basal granule of 9x3 microtubules Nucleus Chromosomes 2x22 + sex chomosomes composed of DNA (2.5x109 base pairs), histones contain genes, promoters, enhancers, junk Nucleolus site of RNA synthesis by transcription Envelope with perinuclear cisterns very permeable to allow RNA out c. Explain mechanisms of transport of substances across cell membranes. Diffusion rate determined by (Fick’s Law) chemical gradient electrical gradient cross-sectional area of boundary thickness of boundary Donnan Effect non-diffusible ions affect diffusion of other ions. ratio of diffusible cations between compartments equals inverse ratio of diffusible anions. Solvent drag unimportant effect. Solvent bulk flow carries solute. Filtration rate determined by pressure gradient surface area of boundary permeability of boundary Osmosis solvent molecules cross a membrane to a region of higher activity of a non-diffusible solute. P=nRT/V Carrier-mediated transport facilitated diffusion transport usually of large, non-ionized molecules down a concentration/electrical gradient across the cell membrane via membrane proteins. e.g. glucose uptake active transport transport of molecules or ions against of concentration or electrical gradient, usually mediated by ATPase proteins in the cell membrane e.g. Na+-K+ ATPase, Ca2+ ATPase, H+-K+ β ATPase ATP α transports 3 Na+ out of and 2 K+ into cells inhibitied by cardiac glycosides composed of 2 α (95 kd binds ATP and digoxin) and 2 ß 3Na+ (40 kd glycoprotein) subunits. Na+ binding is associated with phosphorylation generates a membrane potential rate-limited by intracellular Na+ responsible for most of BMR symport transport coupled to an electrical or chemical gradient e.g. Na+-glucose exchange inmucosal cells, Ca2+-Na+ exchange in cardiac muscle Transport of large molecules Cellular metabolism 1.A.2 James Mitchell (December 24, 2003) proteins and hormones are commonly transported by exo- or endo-cytosis d. Explain the Gibbs-Donnan Effect. e. Outline the role of cellular receptors and the function of secondary messengers within the cell. f. Outline the sources of energy available to cells through metabolic processes. Sources of energy High energy phosphate compounds: ATP, phosphorylcreatine, GTP, CTP, UTP, ITP Thioesters of Coenzyme-A: acetyl-CoA (≡ 1 ATP) Reduced coenzymes: NADH, NADPH (≡ 3 ATP) via flavoprotein-cytochrome system H2 donor, NAD+, FAD, Co Q, Cyt B, Cyt c1, Cyt c, Cyt a, Cyt a3, O2. Carbohydrates Dietary sugars → di- and mono-saccharides in gut → circulating glucose, fructose and galactose (→ glucose) → intracellular glucose ↔ glucose 6-PO4 (- ATP) Embden-Meyerhof pathway glycogen ↔ n glucose 1-PO4 ↔ glucose 6-PO4 ↔ fructose 6-PO4 ↔ fructose 1,6 diPO4 (-ATP) ↔ dihydroxyacetone PO4 (↔ glycerol) + phosphoglyceraldehyde → → pyruvate (+ 2 ATP, 1 NADH) Hexose-monophosphate shunt glucose 6-PO4 ↔ 6-phosphogluconic acid → pentoses → fructose 6-PO4 or phosphoglyceraldehyde Τricarboxylic acid cycle pyruvate + CoA → acetyl-CoA (+ 2H + CO2) … + oxaloacetic acid → citric acid → → → oxaloacetic acid + 8H + 2CO2 net yield = 10H → 5NADH → 15ATP Aerobic glycolysis proceeds via the Embden-Meyerhof pathway and TCAC for 38 ATP per glucose molecule. Anaerobic metabolism relies on the Embden-Meyerhof pathway only, yielding 4 ATP per glucose molecule less one for the phosphorylation of fructose 6-PO4 and one more if glucose 6-PO4 is generated from circulating glucose. The generation of NAD+ required is via the conversion of pyruvic acid to lactic acid, generating an “oxygen debt”. Control of glucose metabolism is regulated by β adrenergic receptors which promote glycolysis via cAMP, protein kinase, phosphorylase kinase and phosphorylase a as well as inhibition of glycogen synthase when it is phosphorylated. This causes a rise in blood glucose and lactate largely arising from glycolysis in liver and muscle respectively. α adrenergic receptors which activate phosphorylase kinase via intracellular Ca2+. Glucagon which stimulates phosphorylase in liver only, causing a rise in blood glucose without lactate. Insulin g. Explain the ways in which cells use energy for the various cellular processes. h. Describe the composition of intracellular fluid and its regulation including the role of the sodium-potassium pump. ECF (20%) ≈ estuarine water Interstitial fluid (15%) Na+ 143 mEq/l K+ 4 Ca2+ 5 Cellular metabolism ClHCO3HPO421.A.3 117 mEq/l 27 2 James Mitchell (December 24, 2003) Mg2+ 3 SO421 org acid 6 protein 2 plus H2CO3 and non-electolytes Plasma (5%) Na+ 152 mEq/l K+ 5 2+ Ca 5 Mg2+ 3 Cl113 mEq/l HCO327 2HPO4 2 SO421 org acid 6 protein 16 plus H2CO3 and non-electolytes Transcellular fluid (small) CSF, aqueous humor, GIT contents etc. ICF (40%) Very rough concentrations: Na+ 14 mEq/l PO42113 mEq/l + K 157 HCO310 2+ Mg 26 protein 74 plus H2CO3 and non-electolytes i. Describe the role of G-proteins. j. Describe the general response to injury. Cellular metabolism 1.A.4 James Mitchell (December 24, 2003)