Advanced Chemistry--Chapter 21: Nuclear Chemistry
advertisement

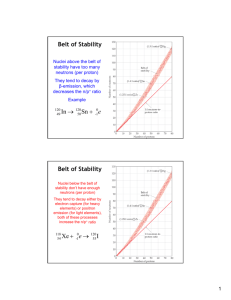
AP Chemistry--Chapter 21: Nuclear Chemistry I. Radioactivity A. Some Terms 1. nucleon--protons and neutrons; particles within the nucleus 2. isotopes--atoms with the same atomic number but different mass numbers (because number of neutrons is different) 3. nuclide--nucleus with a specified number of protons and neutrons 4. radionuclides--nuclei that are radioactive 5. radioisotopes--atoms containing radionuclides B. Nuclear Equations 1. Some nuclei are unstable and spontaneously undergo radioactive decay in which particles and electromagnetic radiation are emitted 2. When representing radioactive decay in equation form, the sum of the mass numbers and the sum of the atomic numbers is the same on both sides of the equation C. Types of Radioactive Decay 1. alpha radiation--a stream of 24 He nuclei is emitted, known as alpha particles 2. beta radiation--a stream of high speed electrons represented by 10 e or 0 1 II. Note: Highlighted items are the concepts addressed on the AP Exam, other information given is to compliment the subject and outline information presented in the text in Chapter 21. ; beta emission is equivalent to the conversion of a neutron ( 01 n ) to a proton ( 11 p or 11 H ), thereby increasing the atomic number by one 3. gamma radiation--also called gamma rays, consists of high-energy photons, or in other words, electromagnetic radiation of very short wavelength; gamma radiation changes neither atomic number nor mass number and is represented by 00 or just 4. positron emission--a positron is a particle that has the same mass as an electron but an opposite charge and is represented by 10 e ; when a positron is emitted, the atomic number decreases by 1 and has the effect of converting a proton to a neutron 5. electron capture--the capture by the nucleus of an inner-shell electron from the electron cloud surrounding the nucleus, shown on reactant side of equation Patterns of Nuclear Stability A. Neutron-to-Proton Ratio 1. At close distances, the strong nuclear force (sometimes just called strong force) holds nucleons together in the nucleus 2. Certain ratios of protons and neutrons in the nucleus leads to stability of the nucleus 3. This neutron-to-proton ratio of stable nuclei increases with increasing atomic number 4. See chart, pg. 997, Zone of Stability a. The Zone of Stability ends at element 83, bismuth b. All nuclei with 84 or more protons are radioactive III. 5. The type of radioactive decay that a particular radionuclide undergoes can be determined from its neutron-to-proton ratio (usually!) a. nuclei above the zone of stability will tend to emit beta particles to decrease their neutron-to-proton ratio b. smaller nuclei below the belt of stability will tend to undergo positron emission; larger nuclei below the belt of stability will tend to undergo electron capture (both increase neutron # and decrease proton #) c. nuclei with atomic number 84 will emit alpha particles to move diagonally toward the belt of stability since alpha emission lowers neutron and proton # by 2 B. Radioactive Series 1. Sometimes nuclei cannot gain stability by just a single emission so a series of nuclear reactions occurs that end with a stable nucleus--this is known as a radioactive series or nuclear disintegration series 2. Three that occur in nature: a. uranium-238 to lead-206 (pg. 1001) b. uranium-235 to lead-207 c. thorium-232 to lead-208 C. Further Observations: two more guidelines to predict nuclear stability 1. nuclei with 2, 8, 20, 28, 50, 82 protons or 2, 8, 20, 28, 50, 82, 126 neutrons are more stable than nuclei that do not contain these numbers (referred to as magic numbers) 2. nuclei with even numbers of both protons and neutrons are generally more stable that those with odd numbers of nucleons Nuclear Transmutations A. Nuclear transmutations are nuclear reactions in which one particle is converted to another particle by being struck by a neutron or another nucleus B. Nuclear transmutations are abbreviated by listing in order the target nucleus, the bombarding particle, the ejected particle, and the product nucleus; in this notation, neutrons are abbreviated n, protons are abbreviated p, and alpha particles are abbreviated C. Using Charged Particles 1. Transmutations can be achieved by bombarding a nucleus with a charged particle 2. Since the particle has a charge, it experiences a repulsion force from the nucleus it is being aimed at. To overcome this charge, the particle has to be traveling very fast. 3. The higher the nuclear charge on either the projectile (bombarding particle) or the target, the faster the projectile must be moving to bring about a nuclear reaction 4. Particle accelerators are used (pg. 1006, 1026), which operate using strong magnetic and electrostatic fields D. Using Neutrons 1. Neutrons can also be used to bring about a transmutation 2. Since neutrons have no charge, they do not need to be accelerated IV. V. 3. Most synthetic isotopes used in medicine and research are made using neutrons as projectiles E. Transuranium Elements 1. All elements beyond uranium on the periodic table are called transuranium elements 2. These are all man-made (not found in nature) by artificial transmutations, they are all radioactive, and some are very “short-lived” Rates of Radioactive Decay A. Half-life 1. Half-life is the time required for half of any given quantity of a substance to decay 2. Each isotope has its own characteristic half-life 3. Half-lives are unaffected by external conditions such as temperature, pressure, or state of chemical combination; they are also unaffected by catalysts B. Dating 1. Carbon-14 a. In living beings, the ratio of carbon-14 to carbon-12 is constant b. When a being dies, the ratio of carbon-14 to carbon-12 decreases (carbon-12 amount increases) c. This change in ratio can be used to determine the age of something that was once living 2. Other isotopes can also be used; for example, the ratio of lead-206 to uranium-238 can be used to determine the age of rocks C. Calculations Based on Half-life 1. Rate kN In this equation, rate is the rate at which a sample decays, also called its activity. Rate is measured in decays or nuclei disintegrations per time. N is the number of radioactive nuclei in the sample and k is the decay constant. What would the decay constant be measured in?? N kt In this equation, t is the time interval of decay, k is the 2. ln t N0 decay constant, N0 is the initial number of nuclei at time zero, and Nt is the number of nuclei remaining after the time interval. Note that the mass N ratio or activity ratio can be substituted into the above equation for t . N0 0.693 3. k In this equation, the relationship between the decay t1 / 2 constant, k, and the half-life, t1/2, is given. Detection of Radioactivity A. Photographic film has long been used to detect radiation; the greater the extent of exposure to radiation, the darker the area of the developed negative B. The Geiger Counter is designed to detect the ions and electrons produced by radiation; a type of probe detects the charged particles and the counter "clicks" VI. C. A scintillation counter detects visible light emitted when radiation strikes a phosphor (a substance that, when excited by radiation, gives off visible light as electrons return to their lower-energy states) D. Radiotracers 1. Because all isotopes of an element act the same way in chemical reactions, small amounts of radioactive isotopes can be mixed with more abundant stable isotopes of the same element and go through the same reactions 2. This fact is used to trace an element through complex chemical processes to find out more about them; the element can be detected at each step using methods described earlier 3. The added radioisotope is called a radiotracer Energy Changes in Nuclear Reactions A. Energy changes in reactions are based on Einstein's famous E = m • c2 equation 1. In the above equation, E is energy, m is mass, and c is the speed of light 2. Because the speed of light is such a large number, small changes in mass result in large amounts of energy 3. In chemical equations, the change in mass is so small that these reactions are assumed to follow the law of conservation of mass a. In actuality, if a system loses mass, it loses energy (exothermic) b. Also, if a system gains mass, it gains energy (endothermic) 4. In nuclear reactions, the change in mass is significant a. The change in mass, m, = mproducts mreactants b. Mass changes are usually calculated per mole so that the amu values of the isotopes can be used for mass Unit Analysis of E = m • c2 5. Nuclear Binding Energies a. It was found that the masses of nuclei are always less than the masses of the individual nucleons of which they are composed b. The mass difference between a nucleus and its constituent nucleons is called the mass defect c. The mass difference arises from the energy that is used to overcome attractive forces to form the nucleus (another way of understanding this is that mass difference comes from the energy that must be added to a nucleus in order to break it into separated protons and neutrons) d. The energy required to separate a nucleus into its individual nucleons is called the nuclear binding energy e. On the chart, pg. 1014, note that the binding energy per nucleon increases dramatically through the very low mass number particles and elements, reaches a peak at mass number 50-60, and then gradually decreases through the larger and larger mass numbers VII. VIII. Nuclear Fission A. The above tells us that heavy nuclei gain stability and therefore give off energy if they are fragmented into two mid-sized nuclei; this process is known as fission B. Since fission is an exothermic process, commercial nuclear power plants and the most common forms of nuclear weaponry use fission for their operation C. Three common isotopes used to produce fission are uranium-235, uranium233, and plutonium-239 D. The two different reactions for the fission of uranium-235 are 137 97 1 1. 01 n 235 92 U 52Te 40 Zr 2 0 n 142 91 1 2. 01 n 235 92 U 56 Ba 36 Kr 30 n E. Atomic Bombs 1. The 2-3 neutrons produced in the fission of one uranium atom power the fission of 2-3 more uranium atoms, those 2-3 produce 4-6, those, 8-12 and so on 2. The number of fissions and the energy released quickly escalates and, if unchecked, results in a violent explosion; this type of multiplying reaction is called a chain reaction 3. The amount of fissionable material large enough to maintain the chain reaction with a constant rate of fission is called the critical mass a. the critical mass allows only one neutron to be supplied for the next fission to occur b. a supercritical mass is a mass in excess of the critical mass; this can lead to a nuclear explosion 4. In the structure of a bomb, two sub-critical masses are slammed together using chemical explosives to get a supercritical mass F. Nuclear Reactors 1. Nuclear power plants usually use uranium-235 in the form of fuel rods as the fissionable material, which is usually enriched to about 3% 2. Control rods are used to absorb neutrons to keep the fission reaction controlled 3. See picture, pg. 1019 4. Storage of spent fuel rods is a current concern a. strontium-90 is a product of the fission of uranium b. it is dangerous and has a half-life of 28.8 years so it needs to be stored for 600 years until it is considered "safe" Nuclear Fusion A. The binding energy chart also tells us that energy is produced when light nuclei are fused to form heavier one B. This is process is called fusion; fusion is what powers our sun C. Among the several fusion processes that are believed to occur are the four below 1. 11 H 11H 12 H 10 e 2. 11 H 12H 23He 3. 23 He 23He 24 He 211H 4. 23 He 11H 24 He 10 e D. Fusion is an attractive idea to generate energy because of the abundance of light isotopes and the fact that products of fusion are not radioactive 1. The problem is that lowest temperature required for any fusion is that needed to fuse deuterium ( 12 H ) and tritium ( 13 H ) in the following IX. 4 1 reaction: 12 H + 13 H 2 He 0 n 2. This reaction requires 40,000,000 K which has only been generated using atomic bombs (this is how thermonuclear or hydrogen bombs operate), not such a good idea for your local power plant 3. We as of now have only been able to safely generate 3,000,000 K using a tokamak fusion test reactor, which uses strong magnetic fields to heat and contain the reaction Biological Effects of Radiation A. Ionizing and Nonionizing Radiation 1. Ionizing radiation is radiation that removes an electron from an atom or molecule and is far more harmful to biological systems 2. Ionizing radiation produces a free radical, OH, from water which disrupts necessary chemical reactions 3. Obviously, nonionizing radiation is radiation that does not cause ionization B. Body tissues most affected by radiation are those that reproduce quickly, such as bone marrow, blood-forming tissues, and lymph nodes; principle effect from extended exposure to radiation is to induce cancer C. Radiation Doses 1. Units of activity a. the becquerel (Bq) is the SI unit of activity; 1 bq is one nuclear disintegration per second b. the curie (Ci) is also used; 1 Ci = 3.7 x 1010 disintegrations per second 2. Units of exposure a. the gray (Gy) is the SI unit of absorbed dose and corresponds to the absorption of 1 J of energy per kg of tissue b. the rad (radiation absorbed dose) corresponds to the absorption of 0.01 J of energy per kg of tissue; rad used most often in medicine c. 1 Gy = 100 rads 3. Since not all radiation forms are equal in strength of damaging biological materials, a correction is made to get a better idea of dosage a. the number of rads is multiplied by the RBE (relative biological effectiveness) for the type of radiation exposure b. the result is the number of rems (roentgen equivalent for man) 4. The SI unit of effective dosage is the Sievert (Sv); 1 Sv = 100 rem (rem used in medicine) 5. Table and chart, pg. 1025, gives some idea of what we receive a. an exposure of 600 rem is fatal in most humans b. a dental X-ray, by comparison, is about 0.0005 rem (0.5 mrem) c. the average exposure for a person in one year due to all natural sources of ionizing radiation (called background radiation) is about 0.360 rem (360 mrem) D. Radon 1. Radon-222 is a product of the decay of uranium-238, and polonium-218 is a decay product of radon; all of these are radioactive a. both radon and polonium have relatively short half-lives, so they decay quickly increasing radiation received b. also, both are alpha particle emitters; alpha radiation has a RBE 10 times that of beta and gamma 2. Homes made with rocks with high uranium content, or homes located in areas where uranium levels are high can be at risk of having radon levels above the EPA recommended 4pCi per liter of air