The melting-curve of neon to 200 kg/cm2
advertisement

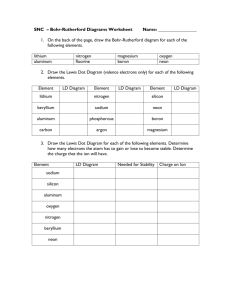
Physics. - The melting-curve of neon to 200 kglcm 2 • By W. H . KEESOM and J. H. C. LISMAN. (Communication No. 224b from the KAMERLINGH ON NES Laboratory at Leiden). (Communicated at the meeting of April 29, 1933). Summary . The melting·curve of neon was determined in the range from the triple-point to 198.i kgJem 2 and 27.i2° K. We used a eryostat in which the hydrogen boils under a pressure of some atm. § 1. Introduction . In 1929 SIMON , RUHEMANN, and EOWAROS 1) determined the melting-curve of neon up to 4900 kg/cm 2 and 69.1 0 K. Our apparatus, with which we measured the melting-curve of hydrogen 2), enabling us to makè more accurate measurements, we decided to carry out a new investigation of the melting-curve of neon. Our measurements cover the range from the triple-point to 198.4 kg /cm 2 and 27.42 0 K. § 2. Method and apparatus. The method followed 3) and the cryostat used 4) were described in previous papers. Temperatures were measured with the platinum thermometer Pt 24' and pressures were read on a metal manometer which had been calibrated with a pressure balance. The neon was kindly supplied by the PHILIPS' Lampworks, Eindhoven 5) and had been purified thoroughly by freezing with solid hydrogen 6). The apparatus was filled as follows: some 15 L of very pure neon was frozen , by means of liquid hydrogen, in a small copper tube, connected with the apparatus. After dosing the cock through which the neon had been supplied, the tube was brought at room temperature, while the neon evaporated into the apparatus, giving an initial pressure of some 25 kg/cm 2 • 1) P. SIMON, M . RUHEMANN and W . A. M. EDwARDs. Zs. f. pbys. Chem . B 6. 331. 1930. 2) W. H. KEESOM and J. H. C . LISMAN, These Proeeedings 35. 607, 1932. Comm. Leiden NO. 221a. 3) H. KAMERLINGH ONNES and W . VAN GULIK. These Proceedings 29, 11 Si, 1926. Comm. Leiden NO . l84a . i) W. H. KEESOM and J. H. C. LISMAN, These Proeeedings 3., 602, 1931. Comm. Leiden NO. 213f. ~) We are greatly indebted to the PHILIPS' Lampworks and to chem. doets. H. PILIPPO, Chief Engineer of tbis firm. for their kind assistance. 6) We render our eordial thanks to Messrs. H. VAN DIJK and J. A. VAN LAMMEREN. asslstants at the KAMERLINGH ONNES Laboratory, for purifying the neon. 379 Por the measurements of the two highest points of the melting-curve. a small quantity of neon had to be added. As for neon the heat of fusion per cm 3 is much greater than e.g. for hydrogen 1). melting and solidification went on much more slowly. 50 for getting an accurate determination of each melting point. several measurements were necessary now. § 3. The results of the measurements are given in table I. We include the triple-point previously measured in this laboratory. The pressures are given in kgjcm 2 reduced to normal gravity. Fig. 1 shows the melting-curve. 2S <'Ii,m' .. KEEsOM AND LISMAN . Q SII\"ON, RUHEMANN AND E~RDS. / 2 00 V / j" sa 00 p I / / V 27 2S Fig. I . The agreement with the measurement of the triple-point by CROMMELIN and GIl3S0 N is good. 50 is also the agreement with CLUSIUS' value of the normal melting point : 24.59° K. 2) SIMON, R u H EMANN and EDW ARDS 3) found a melting-pressure of 140 I) Cf. W . H. KEESOM and H. KAMERLINGH ONNES, These Proceedings 20, lOOD, 1918. Comm. Leiden NO. 153a. 2) K. CLUSIUS. Zs. f. phys. Chem. B ., 1. 1929. 3) F . SIMON, M . RUHEMANN and W. A . M. EOWAROS, 1. c. 380 kg/cm 2 at 26.8° K. This point does not agree very weil with our curve. hut it should he considered that the relative accuracy of SIMON and his cowor~ TABLE I. T oK. 24.57 1) I I TABLE Il. T OK. p kg/cm1. 0.43 p kg/cm 2• 25 28.1 24.87 19 . 5 25.5 61.9 25 . 27 46 . 4 26 95.4 25.79 81.7 26 . 5 130.2 26 . 11 102.7 27 166.9 26.57 135 . 2 27.5 204.5 26.60 5 136.5 28 242 . 7 27 .01 s 168.0 27.42 198 . 4 kers ' measurements is smaller at these pressures than at higher ones. We have chosen SIMON and GLATZEL's formula 2) : IOlog (a + p) = C IO/og T + b, wh ere a. b. and care constants. to represent our results. Using three points, we Eind : a = 728.8 b = -0.16875 c = 2.18038. The formula does not represent the measurements exactly; the differ~ ences hetween the ohserved and the calculated pressures are systematical. With th is formula. taking these deviations into account, we calculated the melting ~pressures of Table 11 . . I) Triple ·point : C. A. CROMMELIN and R. O. GIBSON, These Proceedings 30, 362. 1927. Comm. Lelden NO. 185b. 2) F. SIMON and G. GLATZEL. Zs. f. anorg . u. allgem. Chem. 178, 309, 1929.