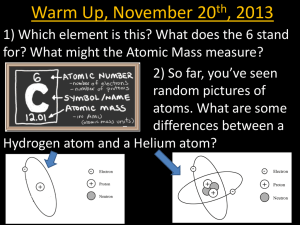
Liquefaction of gases and discovery of superconductivity: two very
advertisement

Revista Brasileira de Ensino de Fı́sica, v. 33, n. 2, 2601 (2011) www.sbfisica.org.br História da Fı́sica e Ciências Afins Liquefaction of gases and discovery of superconductivity: two very closely scientific achievements in low temperature physics (Liquefação de gases e a descoberta da supercondutividade: dois feitos cientı́ficos na fı́sica de baixas temperaturas intimamente relacionados) Simón Reif-Acherman1 School of Chemical Engineering, Universidad del Valle, Sede Meléndez, Cali, Colombia Recebido em 15/11/2010; Aceito em 15/3/2011; Publicado em 3/6/2011 Liquefaction of helium and the discovery of superconductivity are two of the most striking developments in low temperature physics. The fact that both were carried out in the laboratories of Kamerlingh Onnes at Leiden is not mere coincidence; the first one was indispensable for the researches that led to the second one. On the same way, liquefaction of helium was the consequence of several decades of efforts addressed to the process for liquefy the so-called then ‘permanent gases’. A whole study of this remarked subject must then include developments that extended, in his decisive step, more than a half of a century and that connect researches of many scientists throughout several European countries. Keywords: liquefaction, superconductivity, electrical conductivity, electrical resistance, helium, electron theory. A liquefação do hélio e a descoberta da supercondutividade são dois das mais surpreendentes desenvolvimentos da fı́sica de baixas temperaturas. O fato que ambas ocorreram no laboratório de Kammerlingh Onnes em Leiden, na Holanda, não é mera coincidência: o primeiro foi indispensável para que os pesquisadores pudessem chegar à segunda. Do mesmo modo, a liquefação do hélio culminou após décadas de esforços em liquefazer os chamados “gases permanentes”. Um estudo mais completo deste assunto requer a discussão de estudos que se estenderam por mais de 50 anos, ligando pesquisadores de diferentes paı́ses europeus. Palavras-chave: liquefação, supercondutividade, condutividade elétrica, resistência elétrica, hélio, teoria de elétrons. 1. Introduction The study of the remarkable properties exhibited by the matter in the vicinity of the absolute zero became one of the main research subjects carried out in the second half of the XIX century and the first decades of XX century in low temperature physics. The discovery of new phenomena derived from these researches decisively contributed to the dramatic changes that affected and characterized a wide group of scientific and technological disciplines on the following times. Superconductivity, whose first centenary is commemorated this year, is, very probably, the most striking of them. The observation of the abrupt drop in the electrical resistance of mercury wire to an immeasurably small value as it is cooled below at a certain temperature was the culminating development of a entire research program carried out by the by the Dutch physicist Heike Kamerlingh Onnes at his cryogenic laboratory at Leiden, which had begun about three decades earlier. 1 E-mail: sireache@univalle.edu.co. Copyright by the Sociedade Brasileira de Fı́sica. Printed in Brazil. One of the most important sources for those changes was helium, the chemical element first detected by the French astronomer Pierre-Jules-César Janssen (18241907) in 1868 in India through a bright yellow line he observed in spectroscopic study of the chromosphere during a total eclipse of the sun [1], and latter recognized as different to those then known by the astronomer Joseph Norman Lockyer (1836-1920), in collaboration with the English chemist Edward Frankland (1825-1899) [2]. Once some the first sources of helium were identified in earth, and along the full century, its unique physical and chemical properties, such as low boiling point, density and solubility, and inertness, respectively, progressively allowed a lot of remarked developments in so diverse fields, as for example astronomy, cryogenics, medicine, electronics, power generation, communications, energy storing and transportation systems, among others. The liquefaction of helium by first time on July 10, 1908 not only put end to a race that involved scientists 2601-2 from different countries with the same objective, but opened up a whole new field of research. The first part of this article is mainly concentrated in the researches carried out at the University of Leiden, and focuses on the different details that characterized the initial steps of the whole program of liquefaction of those so-called ‘permanent’ gases and how each chapter of the full story contributed in different measure to the successful obtaining of liquid helium at Leiden. It is subsequently shown the way how the apparatuses, cryogenic facilities and strategies developed along this program became the indispensable infrastructure that three years later would lead to the discovery of the phenomena of superconductivity. 2. The basic facts and the state-of-art of liquefaction of gases in the beginnings of XX century As whatever successful technology, the closed relationship between academic research and industrial innovation, besides appropriate communication and commercial good sense, were the ingredients for the early developments on low temperature physics. The underlying basic motivations can then be looked from different viewpoints. Several elements contributed to the increasing interest on the very particular behavior of substances at low temperatures at the end of the XIX century and its possible applications. Liquefaction of gases was then the most important experimental tool and source of information to get it. Initial trials with ammonia by the Dutch chemist Martinus van Marum (1750-1837) in 1769 [3], and others with chlorine by the English chemist and physicist Michael Faraday (1791-1867) in 1823 [4], showed the possibility to liquefy a gas only by compression, and varying both pressure and temperature, respectively. The works carried out on the concept of critical temperature, first on its existence by the French physicist Charles Cagniard de la Tour (1777-1859) in 1822-1823, and four and half decades later by the Irish physician and chemist Thomas Andrews (1813-1885) on the clarification of its nature and hence the relationship between the gaseous and liquid states of matter, supplied a theoretical basis to previous successful trials of liquefaction [4]. From a practical viewpoint, the each time more observed necessity about the use of cold in different areas of preservation of foods, as for example fermentation of some brewing industries and intercontinental shipment of meat, together with the growing concern about the utilization of natural ice from frozen polluted sources of water, had stimulated the development of new equipments for making artificial ice, such as vapor compression and absorption refrigerators [5].On the other hand, the increasing unpopularity of known refrigerants as Reif-Acherman ammonia and sulfur dioxide at that time, because of their hazardous or toxic nature, had began to arouse scientific interest to turn the attention to utilize new compounds for this just purpose, which coincide with the recent discovery of those we now know as noble gases. The possibility to evaluate quantitatively different properties of substances for confirming or rejecting emerging or established scientific theories, or for implementing new and more accurate measuring instruments for each one of them, became an additional motivation for studying these new ranges of temperature. Electrical resistance, as it is shown below, was one of these properties [6]. The definitive step had happened in 1877 with the practically simultaneous but independent liquefaction of the first “permanent” gas, oxygen, by the French mining engineer Louis Paul Cailletet (1832-1913) [7] and the Swiss physicist Raoul Pierre Pictet (18461929) [8], using two different methods. Whereas Cailletet’s involved high compression, then mild cooling, and finally a sudden decrease in pressure, Pictet’s consisted of a series of stages during each of which a different gas was liquefied by exploiting the corresponding thermodynamic properties of the preceding stage. Very quickly other European scientists, as the Polish Zygmunt Wróblewski (1845-1888) and Karol Olszewski (1846-1915) at the Chemistry Department of the Jagiellonian University in Cracow, took interest in the same subject, tried some improvements in the equipment and the operating techniques, and extended the whole field of research. Although different gases were studied, the interest was mainly focused in two of them: hydrogen and helium. 3. The liquefaction of hydrogen as starting point and a false claim of priority The liquefaction of oxygen put end point to the concept of “permanent gases”, and after that nobody doubted about the possibility to turn other gases into the liquid state. Hydrogen was the following step. The efforts were then focused on substantial improvements over the original Pictet’s method, then known as the cascade process. The researches followed then two simultaneous and dependent on each other lines: the quest of each time more lower temperatures, and investigation of properties of substances in these new ranges. The main protagonists of the hydrogen’s chapter, that by first time -although mistakenly- involved helium, were the Dutch professor of experimental physics and meteorology at the University of Leiden Heike Kamerlingh Onnes (1853-1926) - Fig. 1 -, and the Scottish professor of chemistry at the Royal Institution in London -later Sir- James Dewar (1842-1923). They had the same ultimate objective, but great differences in Liquefaction of gases and discovery of superconductivity: two very closely scientific achievements in low temperature physics their investigative attitudes and motivations. 2601-3 different properties of matter was his main objective. His words reveal a more scientific interest on the subject. “The territory of low temperatures,” he said, “has always tempted the experimenters. . . The arctic regions in physics incite the experimenter, as the extreme north and south [poles] incite the discoverer” [13]. He based the whole program for the liquefaction of gases on the experimental verification of the theories of his compatriot and friend Johannes Diderik van der Waals (18371923), which served him as motivation to seek lower and lower temperatures to investigate the properties of matter. The publication of van der Waals’s thesis on the continuity of the states [14], five years before the liquefaction of oxygen and almost completely ignored by Dewar, not only had showed the first theoretical breakdown of the Boyle’s law, but supplied a general frame to the procedure for the development of the cryogenic researches and the design of the corresponding equipment. Figura 1 - Heike Kamerlingh Onnes (1853-1926). Credit: Courtesy of the Kamerlingh Onnes Laboratory, Leiden Institute of Physics, University of Leiden. Dewar, who got involved in low temperature research around the middle of 1880’s, didn’t see the theory as complement to the experiment into the physical research, as it is shown by the fact that no one theoretical paper can be found through the extensive group of publications on liquefaction or other subjects along his full career. His was an absolutely practical interest, directed to the identification of new techniques for lowering the temperature and purifying gases [9]. In 1892 he was able to use his invention of the double walled silver glass vacuum vessel that actually bears his name to store cryogenic liquids for some long periods of time. On May 10, 1898 he collected by first time approximately 20 cm3 of liquid hydrogen in five minutes by using the Joule-Thompson effect at adapting an interesting process developed by the German engineer Carl Paul Gottfried von Linde (1842-1934) [10]. Based on wrong analysis of previous information and on experimental restrictions [11], Dewar concluded that hydrogen and helium have almost identical boiling points, and publicly communicated to the Royal Society, and to his main “rival” colleague, the simultaneous liquefaction of both compounds (Fig. 2). It was shown very quickly that what Dewar thought was liquid helium were really traces of impurity, and that the objective for liquefy helium must to wait for a next opportunity and other circumstances. As far as his publications reveal, and probably due to his remarked proud and polemic personality, he never admitted explicitly his mistake in public. Kamerlingh Onnes, by his side, had a very different motivation and philosophy [12]. The study of the general character of the influence of low temperatures on Figura 2 - Telegram from Dewar to Kamerlingh Onnes (1898). Credit: Courtesy of the Museum Boerhaave. 4. The work at the cryogenic laboratory in Leyden At a fully reconstructed laboratory, superbly equipped with the sophisticated apparatus he required, and adapting what he considered the best ideas of other researchers, Kamerlingh Onnes embarked on two main purposes [15]. The first one was an extensive and systematic program to measure the volumetric properties of a great variety of pure substances over the widest possible range of conditions, which, in light of van der Waals’s law of corresponding states, might enable him to predict their properties at low temperatures [16]. The development of his virial equation of state, with 2601-4 twenty-five parameters dependent in a different way on temperature, helped in this purpose [17]. The equation, which expresses the product of the pressure of a gas and its molar volume as a finite polynomial series expansion in powers of the inverse molar volume, was a tool for understanding better the real behavior of gases. The second purpose was the production of large quantities of liquefied gases that he required for the experiments, work that was preceded by a very careful process of purification of each substance under study; for the moment there was no matter that it seemed that he was behind the achievements of his ‘rivals’. The laboratory had been methodically built stepby-step, and the whole process corresponded with an improved version of the original cascade method with two cycles used by Pictet to liquefy oxygen, which progressively included a larger number of cycles [18]. In each cycle a different gas circulated. It consisted of three basic elements, a condenser or liquefier, a vessel in which the liquid was evaporated at low pressure, and one or more vacuum pumps, which evacuated the gas from the evaporation vessel and compressed into the condenser. According to the arrangement, each cycle operated at a lower temperature than the preceding one. So, the evaporation vessel on one cycle simultaneously functioned as the condenser of the next one. The circulation of the fluid through the system was simple: the liquefaction by compression of the gas circulating at low temperature was followed by evaporation at low pressure, being the vapor pumped back to the compressor, after which the cycle started again. With the evaporation, the reached temperature in the lowpressure chamber was less than that corresponding to the critical point of the fluid used in the following cycle (Fig. 3). Kamerlingh Onnes inspired the choice of the substances of the two first cycles on previous experiences by Cailletet. By 1894 he had constructed a large scale plant with four cycles of methyl chloride, ethylene, oxygen and air, which allowed him to reach temperatures of -193 ◦ C and product fourteen liters of liquid air per hour [19]. After solve between 1896 and 1898 serious obstacles related with the prohibition by the Municipality of Leiden to perform in the laboratory experiments with compressed gases, Kamerlingh Onnes faced with a new objective: liquefy hydrogen. He understood that could not use his cascade method with hydrogen, because its critical temperature is far below the lowest temperature that he could reach at the time, and there was not suitable gas available for a cycle between that with air and the one planned with hydrogen. By applying van der Waals’s law of corresponding states, he was able to predict the properties of hydrogen at those lower ranges of temperature, and, as Dewar did, incorporated the Linde process to his general scheme. In 1906 the laboratory included a hydrogen cycle as fifth cycle, reached a temperature of about -253 ◦ C, and an Reif-Acherman initial production of four liters per hour, which very quickly extended to thirteen. Figura 3 - Diagram for the cascade for liquid gases from 1906. Credit: Courtesy of the Nobel Foundation. 5. The successful liquefaction of helium Although it was true that air and hydrogen were liquefied at Leiden seventeen and eight years, respectively, after these events happened by first time, the Laboratory was at the beginning of XX century in a far more advantageous position than those similar in other European countries. The difference was based on three main aspects: a more careful scientific planning, an installation with equipment infrastructure capable of producing easily large quantities of liquid air and liquid hydrogen, and a remarkable diplomatic skill put at service of solving all those difficulties different to those strictly technical. This last distinctive fact allowed Kamerlingh Onnes to solve problems that ranged from getting funds or different supplies to unexpected events that in some way could retard the activities in the Laboratory. By 1895 sources of helium were found on earth. Working at the Washington Laboratory of the Geological Survey, the American chemist William Francis Hillebrand (1853-1925) observed the evolution of a gas at treating powder of the mineral uraninite with sulphuric acid [20]. According to routine analytical procedures, he thought the compound was nitrogen. Sir Liquefaction of gases and discovery of superconductivity: two very closely scientific achievements in low temperature physics William Ramsay (1852-1916) repeated later the experiments, and sent samples to Lockyer and Sir William Crookes (1832-1919), who spectroscopically found absolute coincidence with the yellow line given by the helium of the sun [21]. The compound was independently and almost simultaneously discovered in other uranium mineral, cleveite, by the Swedish chemist Per Theodor Cleve (1840-1905) and his student Nils Abraham Langlet (1868-1936) [22]. Although in 1905 the American chemists Hamilton Perkins Cady (1874-1943) and his student David McFarland (1878-1955) discovered what would be the first industrial source in the extraction from natural gas [23], the available amounts of helium were definitively limited. Besides technical facts, this was one of the main obstacles for the liquefaction. In 1896 Olszewski did the first attempt to liquefy helium. The substance was obtained from Ramsay [24]. The only marked difference between the two series of experiments he carried out was the refrigerant he used; in the first one he utilized liquid oxygen, and in the second one liquid air. By the evaporation of the liquid refrigerant under a pressure of 10 mm Hg, the lowest temperature he reached was around -220 ◦ C. Even at this temperature, and with a pressure of 140 atmospheres, the following sudden expansion to even atmospheric pressure didn’t give him any evidence of liquefaction. He tried again nine years later with the same method, but this time with helium extracted from a radioactive mineral called thorianite, recently discovered in Ceylan, which was then considered the richest source of this gas (about 9-10 cubic centimeters of helium per gram), and using liquid hydrogen as refrigerant [25]. In spite of work at more extreme conditions, -259 ◦ C and 180 atmospheres, he was again unable to get successful results. Understanding that the lack of the financial means necessary to build the appropriated apparatus he would like to get was an obstacle that he could not solve, and still with doubts about the real possibility to liquefy this gas, Olszewski definitively desisted from his effort. Halfway between the two Olszewski’s trials other persons made also clear their interest on the subject of liquefaction. In a Bakerian lecture delivered in 1901 Dewar did reference to some unsuccessful experiments he carried out performing a similar procedure to that by his Polish colleague [26]. The source for helium was now the gas evolved from the water of the King’s well at Bath, a little English city built in the mouth of an extinct volcano, located just outside of Bristol in the country’s southwest region. A look to some pages of his laboratory notebooks complements this information [9]. Along approximately three years he worked on the subject, isolating enough helium for his experiments and producing the amount he calculated would require of liquid hydrogen as refrigerant in the equipment. Technical difficulties, bad health, and the little confidence he had in his assistants forced him to eventually give 2601-5 up trying. Independently, the British chemist Morris William Travers (1872-1961) did other unsuccessful attempt. From middle of 1890’s Travers had closely worked with Ramsay in the search of missing elements which the periodic law indicated should exist. In this way, it were discovered other rare gases as neon, krypton and xenon, and some of the properties of the five new gases, including argon and helium, were determined. In 1901 he required liquid hydrogen for the cooling step in the liquefaction process to separate a helium-argon mixture. As Dewar never published a description of his apparatus, Travers found himself in the necessity to build his own with the with the limited resources of a small compressor, a small air liquefier and £50 to cover all additional expenditure, and the only help of the laboratory mechanic who carried out the connecting work. The liquefier operated successfully with only a trial [27]. With this record, and together with his compatriot, the also chemist George Senter (1874-1942), and the French physicist Adrien Jaquerod (1877-1957), he proposed try to find the probable values of the critical and boiling points of helium, and attempt liquefy the gas [28]. Previously Travers himself had unsuccessfully tried to fractionate helium by absorption by the platinum splashed on the walls of a vacuum tube that the German mathematician and physicist Julius Plückner (1801-1868) had designed for the determination of trace elements present in solid state samples through using a glow discharge source [29]. The new research was an appendix to a whole work focused on the relationship between the thermodynamic scale of temperature and the various scales of temperature as it was experimentally determined by means of gas thermometers on one side, and the determination of the vapor pressures of liquid hydrogen at very low temperatures on the other. Hydrogen and helium were then considered the most important thermometric substances. The mineral cleveite was the initial source for obtaining pure helium. The equipment consisted of a compression tube of an apparatus similar to that employed by Cailletet (see Fig. 4). The wider portion, A, was capable of standing a pressure of 80 atmospheres, or more, being the compression apparatus connected to its lower end. It was required a pressure of 60 atmospheres to compress the whole of the helium in the capillary tube B cooled with liquid air, which passed through a rubber stopped E into a small silvered vacuum vessel C filled with liquid hydrogen. There were two gauges gathered with the tube to make the measurements in different ranges of the variable. It was provided a plug of natural wool in the upper part of the tube D to shield the mouth from external radiation. The internal pressure at the vessel was measured by a mercury manometer connected to the tube F . A vacuum vessel H, filled with liquid air, enclosed the tube D. A reduction in the pressure of liquid hydrogen 2601-6 by means of the pumps connected through the tube G, that caused decreasing in temperatures to values that were estimated below to -260 ◦ C didn’t lead, nevertheless, to some evidence of liquid helium. Although it’s true that the experimenters observed the near impossibility to do liquefaction with equipments as used, they didn’t dare to do definitive conclusions about the feasibility in other circumstances. What they definitively brought up was the necessity to do extensive studies on the Joule-Thomson effect of helium over a wide range of temperatures to learn its real response, cooling or heating, at be undergone to free expansion at high or at low temperatures. Figura 4 - Hydrogen liquefier used by Travers. Credit: Ref. [27]. The profound interest Travers had in the promotion of the university scheme in the University College at Bristol, where he worked, and the later offer to take up the post as first Director of the then proposed Indian Institute of Science at Bangalore, finally accepted in 1909, forced him to interrupt his work on liquefaction [30]. After his retirement of the Institute in July 1914 and his arriving back to England he embarked on different activities, and never returned to researches on low-temperature physics. In spite of the helium’s discovery on earth happened in an unplanned way thirteen years after Kamerlingh Onnes undertaken his work at low temperatures, the research leading to its liquefaction had a very special meaning for the Dutch scientist. The study, or ‘attack’ on helium, as he said, was “the boldest attack that can be dreamt of in low temperatures” [31]. Helium allowed him to get several times nearer to absolute zero than was possible with hydrogen. He faced up to the subject of liquefaction of helium in a very similar way as he did with the previous gases. Remaining faithful to his belief about take the van der Waals’s law of corresponding states as a guide of his full research program, he defined as first step the determination of Reif-Acherman helium’s isotherms, particularly for those temperatures that could be obtained by means of liquid hydrogen. As in the past time, he was sure it was the more suitable way to calculate its critical properties, and through them determine the conditions for the liquefaction [32]. The first worry Kamerlingh Onnes had was how to have access to enough quantities of helium. Knowing that Bath springs were a good option (the helium content there was about one-thousandth part of the gas evolved from the water of the thermal springs), he contacted the authorities there inquiring about the possibilities to get it there, but they referred him to Dewar. In the letter that Kamerlingh Onnes sent to the British scientist let him know the state of advance of his investigations and the urgent necessity he had to undertake immediately the determination of the isotherms. Some typical features of Dewar’s personality had to presume that the answer would not be favorable, as indeed it was. Although it is true that the relationship between both scientists was cordial, and that they exchanged information and discussed various difficulties they faced in their experimental research, Dewar was a selfish person that didn’t like generally to collaborate with other scientists and regarded them as his rivals. At his answer Dewar said “It is a mistake to suppose the Bath supply is so great. I have not been able so far to accumulate sufficient for my liquefaction experiments. If I could make some progress with my own work the time might come when I could give a helping hand which would give me great pleasure”. He added that “I have in my own way been engaged on this subject for years and after many misfortunes, and with not little expenditure I have been unable to accomplish my specific object. We both want the same material in quantity from the same place at the same time and the supply is not sufficient to meet our great demands” (cursive is mine) [33]. Kamerlingh Onnes didn’t remain waiting if Dewar would be able to honor his promise, and look for other alternatives. Helium could to be isolated too from monazite sand, a primary ore of the same radioactive origin as thorianite, but composed of several phosphates of many of the rare earth metals along with cerium, lanthanum and thorium. It approximately contained 1-2 cubic centimeters of helium per gram. The original process comprised heating of the mineral to 1000 ◦ C, being the escaped helium subsequently purified by treatment with hot metallic calcium, which absorbs nitrogen and other gases [34]. In 1905 the mineral was temporally mined at North Carolina for uses as source of thorium for incandescent mantles, and Kamerlingh Onnes was able to obtain large quantities of it under favorable conditions thanks to the efforts of his younger brother Onno (1861-1935) who was then Director of the Office of Commercial Intelligence in Amsterdam [35]. Few years later, and taking advantage of the mediation of the American chemist and pioneer of radiochemistry Bertram Borden Boltwood (1870-1927) who Liquefaction of gases and discovery of superconductivity: two very closely scientific achievements in low temperature physics had previously visited the Leiden Laboratory, Kamerlingh Onnes would receive two free of charge invoices of helium gas from the Austrian scientist and industrial Carl Auer von Welsbach (1858-1929), founder of Welsbach Light Co., which produced the mentioned mantles by processing large quantities of thorianite (whose treatment was significantly less expensive), in return of important amounts of the waste of the monazite sand treatment, which still contained thorium) [36]. The next step was the careful and perseverant preparation of enough helium of high purity with the assistance of Gerrit J. Flim (1875-1970), the chief of the technical department of the Cryogenic Laboratory, the glassblower Oskar Kesselring, and four chemists. The basic procedure used at Leiden consisted of heating and purifying monazite at low temperatures in a series of stages that included adsorption on charcoal. The isotherms were ready two years later. According to them, he was able to estimate the helium’s critical temperature around 5 K, or maybe slightly higher. This value calmed him down a little. Along their attempts of liquefaction Olszweski and Dewar had estimated the critical temperature closed to 2 K, and he had predicted a similar value. If it had been a true, liquefy helium was an almost impossible experimental objective. By 1908, Kamerlingh Onnes had obtained 360 liters of helium of high purity, and had been able to produce in his laboratory more than 1500 liter of liquid air, more than enough for the operation of his cascade system. It was necessary include a fifth cycle. He was then focused on the design and latter construction of the helium liquefier and the elimination of the contamination from glycerin, the lubricant he used in his vacuum pumps. The liquefier was a closed imitation of the model of that of hydrogen. All the system had to be reduced to a smaller scale, approximately half size, because the limited availability of helium and the capacity of the pumps. Larger reductions had led to construction problems. One of the more operating difficulties was the simultaneous work of hydrogen and helium cycles. Although it is true that this one must to be the most appropriate option, the installed infrastructure was not enough for it. Based on the unquestionable fact that was more difficult obtain liquid hydrogen than liquid helium in the required quantities, it was decided on one hand allow the latter to circulate, forcing the quantities not liquefied pass several times through the cycle, and on the other have previously prepared and stored large amounts of hydrogen. This decision was later confirmed a very good one, because helium must pass twenty times through the cycle before liquefaction was observed. An undated telegram for Dewar, found among the Kamerlingh Onnes’s papers, and that very probably corresponds to few months before the liquefaction, reveals what at the first time was a fleeting excitement, but some time later turned into frustration. The ob- 2601-7 served solidification of some impurities of helium initially suggested that could correspond to the pure element. The reasons Kamerlingh Onnes found for the misinterpretation in the traces between 0.45 and 0.37 volume percent contained in the gas used in the experiments, suggested him the necessity to improve the purification method. On July 10, 1908, Kamerlingh Onnes was able to produce more than 60 cubic centimeters of liquid helium in an experiment “that bordered on the impossible” [37], lasting more than 14 hours. A detailed and chronological account of the events was related by the scientist [35, 37]. A diagrammatic scheme of the full helium cycle is shown in the Fig. 5. The work began at 5:45 a.m. when were prepared and ready to use 20 liters of liquid hydrogen, which were stored in silvered vacuum glasses. After be sure that there was no contamination in each piece of the full arrangement, it was proceeded at 1:30 p.m. to the filling with liquid air and liquid hydrogen to protect the glasses in the hydrogen and helium cycles, respectively. At 4:20 p.m. helium was circulating through the liquefier (see Fig. 6). Fifteen minutes later the pressure of the helium began to be slowly increased from 80 to 100 atmospheres, and one hour after that the first trial with a sudden expansion to 40 atmospheres occurred. The temperature was then around 6 K. Several new trials were done, and even using the last available bottle of liquid hydrogen still nothing was observed. Figura 5 - Helium’s cycle in the cascade process. Credit: Ref. [35]. That Kamerlingh Onnes had actually produced liquid helium might have passed unnoticed owing to his very low surface tension and consequently the absence of a clear boundary between its gaseous and liquid phases. Thus, all of Kamerlingh Onnes’s careful planning and extensive building of his cryogenic apparatus - the most sophisticated in the world at this time might not have borne fruit, at least not for some time, had someone outside of his team of researchers not noticed that the bottom of his vessel was improperly il- 2601-8 luminated and hence did not permit observation of the gas-liquid boundary layer [38]. However, as he wrote, “After the surface had once been seen, it was no more lost sight of. It stood out sharply defined like the edge of a knife against the glass wall” [35]. The presence of that helium liquid, “that looked almost unreal”, was absolutely confirmed when it had already filled up the vessel. It happened at 7:30 p.m. and the corresponding measurement of temperature, 4.25 K, was the minimum one up till then experimentally reached. A commemorative stone of grey marble related with this event that was placed in the building where then was the Laboratory still stays there (Fig. 7). It was put on by its staff some years later to pay homage to Kamerlingh Onnes. In it are carved the following words: “On this spot, on the 10th of July, 1908, Helium was liquefied for the first time by Dr. Heike Kamerlingh Onnes. The entire staff of the laboratory has presented to him this memorial on the occasion of the 40 anniversary of his professorate. November 11th , 1922”. Figura 6 - Helium liquefier. Credit: Ref. [35]. Reif-Acherman Figura 7 - Commemorative stone for helium’s liquefaction. Credit: Courtesy of the Museum Boerhaave. Credit: Courtesy of RCC Koude & Luchtebehandeling. 6. Superconductivity is on the way It is interesting to note that if it is true that the first part of the whole program of researches in low temperatures at Leiden was specially focused on liquefaction of gases and related areas, the subject of electricity, and specifically the behavior of electrical properties in the same zone, not only arose the interest of Kamerlingh Onnes from the beginnings of his work, but also became one of the parts of what he called “the unity of natural phenomena” [39]. It is clearly revealed in his lecture on the importance of quantitative research on the occasion of his appointment as professor of experimental physics at the University of Leiden in 1882, in which Kamerlingh Onnes devoted an appreciable portion to different electrical subjects [40]. Contrary to what is a widely diffused belief, the researches on the behavior of the resistance of metals as a function of temperature in Leiden, which later would led to the discovery of superconductivity, did not begin in 1908 after the successful liquefaction of helium. The program of a general investigation at Leiden on the relation between the electrical resistance and temperature on the ranges under study there, including different metals, was officially communicated in 1902 [41]. Its initial objectives, as the following papers mainly bearing on electrical measurements showed, were focused on thermometric applications [42, 43]. The state of the art on the subject in those times showed significant advances, which had been specially complemented in the second half of the XIX century thanks to the facilities provided in the range of temperatures opened by the researches on liquefaction of gases. The first significant achievements went back to the Italian physicist Gianbattista Beccaria (1716-1781) [44], and later the English scientist Henry Cavendish (1731-1810), who, through the earliest experiments carried out on the electrical conductivity of various substances [45], were able to show the superior conductivity of metals. The researches were followed by the also British physicist Humphry Davy (1778-1829), who, working in the Royal Institution in London, showed in 1821 by first time that the electrical conductivity of wires of different metals, such as platinum and silver, Liquefaction of gases and discovery of superconductivity: two very closely scientific achievements in low temperature physics decreases with increasing temperature [46]. The experimental researches strengthened with the extensive series of measurements made in 1835 by the Russian physicist H.F. Emil Lenz (1804-1865) in St. Petersburg. Working at temperatures far above 0 ◦ C, he proposed that the electrical conductivity of metals varies quadratically with their temperature [47]. In 1858 the Norwegian physicist Adam Arndtsen (18291919) at the University of Christiania (later Oslo) concluded that it was the electrical resistance and not the electrical conductivity of most of pure metals under study in similar ranges of temperatures which varies linearly with temperature [48]. His paper immediately caught the attention of Rudolf Clausius (1822-1888) at the Polytechnicum (later the Eidgenössische Technische Hochschule) in Zurich, who concluded that “the resistance of a simple metal in the solid state is closely proportional to the absolute temperature” [49]. Subsequent measurements by August Matthiessen (18311870) at the University of London, however, called Clausius’s conclusion into question [50]. A new chapter in the story was opened in 1885 with the report by Cailletet and Edmond M.L. Bouty (18461922) of resistance measurements of several metals (aluminum, copper, iron, magnesium, mercury, platinum, silver and stain) down to the boiling temperature of ethylene (-100◦ C) [51]. Previously bismuth, a metal of commercial interest in the last quarter of the nineteenth century, had been the subject of the researches of the Italian Augusto Righi (1850-1920)[52], the French Anatole Leduc (1856-1937) [53]and the Belgian Edmond van Aubel (1864-1941) [54]. Working separately they published a detailed report of the behavior of its electrical resistance and those of its alloys for moderately low temperatures. The Polish scientist Zygmunt Wróblewski (1845-1888), who together with his compatriot Karol Olszewski (1846-1915) had improved Cailletet’s method in Cracow and had been able to liquefy oxygen, nitrogen, and carbon monoxide [55], extended even more down the temperatures to reach that of solid nitrogen (-200◦ C) [56]. Cailletet and Bouty found a linear dependence of resistance on temperature, while Wróblewski found a much faster variation. That same year, the British physicist Hugh Longbourne Callendar (1863-1930), working under J.J. Thomson in the Cavendish Laboratory in Cambridge, began a systematic series of precision measurements on platinum, that led him in 1899, now at the University of London, to propose a parabolic relationship between resistance and temperature and to become the first one to suggest the possibility that the resistance of “most of the common metals” tends to vanish at a temperature higher than absolute zero [57]. Meanwhile, between 1892 and 1893, James Dewar at the Royal Institution in London and John Ambrose Fleming (1849-1945) at University College, London, had carried out an extensive joint series of resistance measurements, first on eight pure metals and 2601-9 seven alloys [58], and later on fourteen metals [59], down to the temperature of boiling liquid oxygen (-200 ◦ C). Kamerlingh Onnes got involved in experimental work on the subject by first time in February 1906, once hydrogen had been liquefied in his laboratory and its availability let the researches in the new ranges of temperature to be possible. The almost linear behavior of the resistance of platinum at the by then lowest reachable temperatures (that of sublimation of hydrogen -it means 14 K-) recommended its use as a promising secondary thermometer which could be based on calibrated conductivity measurements instead that of helium, which presented its unpractical sizes as additional inconvenience. The first results were reported to the Meeting of the Royal Netherlands Academy of Arts and Sciences (KNAW) four months later. The main objective of this first series of experiments, carried out jointly with his assistant Jacob Clay (1882-1955) was to prove the possible existence of a point of inflexion in the curve representing the resistance as a function of temperature [60]. Platinum was chosen, at first instance, as the standard metal [61]. This first communication was followed by at least four related articles. Two of them showed similar results with gold instead platinum because the possibility to get it in a purer condition [62] and the effect of admixtures on the electrical resistance [63]; the others included the study on the behavior, also as function of temperature, of expansion, a property that was then believed to be related with resistances [64, 65]. From the beginnings the resistances values were tabulated or plotted as ratios of the resistance at the observed temperature to the resistance at a reference value (0 ◦ C or 273.09 K) versus absolute temperature. With the successful liquefaction of helium, the determination of electrical resistance of metals as a function of temperature became a priority for Kamerlingh Onnes. The next objective at Leiden was the corroboration and extension of the new Dewar’s researches [66, 67], and others by Travers and his compatriot Alfred G. C. Gwyer [68], on the subject at lower temperatures. These results not only showed a continuous diminution of the resistance of unalloyed metals as temperature went down, but also would seem to suggest one of two possible trends too; the reaching of a definite asymptotic value, unchangeable with additional decreasing of temperature, or, following a dangerous extrapolation of the available data, the absolute vanishing of resistance at some temperature above absolute zero, or even a negative resistance at this just value. 7. Different theories about behavior of the resistance of metals Kamerlingh Onnes had doubts about what he could expect from the following experiments. Although the formulation of an appropriate theory for explaining the 2601-10 causes by which the phenomenon of contact electricity is produced was one of the objectives of many physicists in the last quarter of XIX century, all those by then existed were still in rudimentary state and left the doors opened to any of the different possible forms of the temperature variation of the resistance (Fig. 8). The theory of the electrical and thermal properties of metals, separately proposed by Eduard Riecke (1845-1915) [69] and Paul Drude (1863-1906) [70], later refined by Hendrik Antoon Lorentz (1853-1928) [71], and based on arguments of classical mechanics, was very probably the most successful. The theory proposed that electrical resistance was a consequence of the thermal agitation between the metal and the conduction electrons, which led to a decreasing of resistance with temperature and to a perfect conductivity of metals at absolute zero. The electric current was treated as a drift of an electron gas under the influence of an electric field. The free movement of the electrons (with a speed depending of temperature) in the spaces between the heavy fixed atoms of the metal with which they exchanged energy by collisions, was restricted in some way in presence of an imposed electric field, being the direction of this latter which define the set up of the electric current. Reif-Acherman (1824-1907) as an inference from the theory of Riecke and Drude [73]. The basic idea here was related with the assumption of a decreasing density of the free electron gas as the temperature approached absolute zero, being zero at this just value with electrons condensing (freezing) onto the atoms. The theory was widely known at Leiden even before its publication at a British journal, as it is revealed with its inclusion in a Jubilee book coordinated by Kamerlingh Onnes on the occasion of the retirement of his early professor Johannes Bosscha (1831-1911) (see Fig. 9) [74]. In practical terms, the model suggested that the resistance of a conductor would reach a minimum somewhere in the curve that represented its behavior, after which it would begin to increase until become ‘infinite’ at absolute zero. Kamerlingh Onnes explicitly referred by first time to the Kelvin’s proposal in his Address as Rector Magnificus in 1904 [13], and it seems that this model was in some way the driver of the related researches in Leiden until 1908. Figura 8 - Possible (qualitative) forms of temperature variation of electric resistance. The constant and temperature independent minimized value of the resistance that characterized the curve I was quickly associated by Kamerlingh Onnes with impurity contents of the specimens under study. He was able to confirm this behavior with platinum as well with several species of gold of different grades of purity. The fact, already known by Matthiessen since 1864 [72], suggested the existence of a constant residual resistance at helium temperatures, with values which were lower the purer the specimens were. The model corresponding to the curve type II emerged in a proposal by William Thompson (later Lord Kelvin) Figura 9 - Thanks letter addressed by Lord Kelvin to Kamerlingh Onnes on occasion of the including of his paper in the Bosscha Jubilee volume (1901). Credit: Courtesy of the Museum Boerhaave. A recently published article, based on the study of the surviving Kamerlingh Onnes’s original notebooks housed at the Boerhaave Museum at Leiden, presents a historical account of the technical details involved in the experiments carried out between 1910 and 1911 [75]. The first successful resistance measurements at helium Liquefaction of gases and discovery of superconductivity: two very closely scientific achievements in low temperature physics temperatures were carried out in December 2, 1910, and reported in February 1911 [76]. Previous experiences had allowed the reaching of a new lower limit for temperature: 1.1 K. The physicists in charge of the experiments were Gilles Holst (1886-1968) and Cornelis Dorsman (1877-1960). Holst (Fig. 10), with some mechanical skills, graduated in mathematics and physics from the Eidgenössische Technische Hochschule in Zurich in 1908 and previously assistant to the German physicist Heinrich Friedrich Weber (1843-1912) in his researches on specific heats, was the main responsible of the electrical measurements by operating a Wheatstone bridge with the galvanometer; Dorsman assisted him with the temperature measurements [77, 78]. Figura 10 - Gilles Holst (paint). Credit: Kamerlingh Onnes Laboratory; courtesy of the American Institute of Physics, Emilio Segrè Visual Archives. The metals under study were again platinum and gold. The new experimental results quickly showed Kamerlingh Onnes the inadequacy of Kelvin’s proposal, and that, instead the resistance passing through a minimum, it approached to zero at a temperature near to the absolute zero as it is schematically indicated by curve III in Fig. 8. Kamerlingh Onnes proposed then the thermal agitation of the oscillators introduced by the German physicist Max Karl Ernst Ludwig Planck (1858-1947) in his quantum theory of radiation as the responsible mechanism for the new results [79]. In contrast to the Kelvin’s proposal, the electrons kept moved around at those lowest temperatures, because it were the oscillators that freeze and not the electrons. By accepting the Planck’s vibrators as explaining element, Kamerlingh Onnes followed the same line of thought used by other researchers also working on behavior of 2601-11 other physical and chemical properties, such as Walther Nernst (1864-1941) and his collaborators Arnold Eucken (1884-1950) and Frederick Alexander Lindemann (1886-1957) in Berlin, with their investigations on specific heat of gases at liquid hydrogen temperatures [80], and Albert Einstein with the recently developed theory of specific heat of solid substances [81]. The researches were to such an extent closely related each other that some author dare to say that, by 1911, “Nernst and his collaborators had thus advanced on the path toward an early ‘discovery’ of superconductivity” [82]. 8. The decisive experiments For the new experiments Kamerlingh Onnes proceeded to implement some changes in his program in order to solve technical inconveniences. On one hand, the older arrangement of helium liquefier and cryostat, very similar to that used in 1908, was significantly modified. In the new assembly, the liquid helium was transferred from the liquefier to a separate helium cryostat, which not only allowed the immersion there of the required measuring instruments, but also the appropriate agitation of the contents and the corresponding keeping of resistances at uniform well-defined temperatures and the handling of larger samples with incensing of sensitivity, accuracy and easiness [83, 78]. Liquid helium handling and transfer became a so ordinary procedure at the physical laboratory at Leiden that it even coming to be nicely caricaturized by members of the staff (Fig. 11). On the other hand, it was decided to use mercury as the metal to be studied instead the previous platinum and gold. The reason was clear: the height of the leveling off of resistivity of metals at lower temperatures that followed the section of its almost linear behavior was clearly concluded dependent of the amount of impurities of the sample of metal used in the experiments. Mercury clearly took shape as the best option because of its facilities for to be repeatedly distilled in vacuum at temperatures between 60 ◦ C and 70 ◦ C to an even higher degree of purity than the other used metals, even gold. There was no matter that mercury was liquid at ambient temperature because it would freeze forming something as a wire once the purified liquid was placed in the glass capillaries, behaving then in the same solid condition as the previously used metals. Figura 11 - Helium procession with Kesselring followed by Kamerlingh Onnes; second from the right is Flim carrying the liquid helium. Credit: Courtesy of the Museum Boerhaave. 2601-12 Using a empirically formula he had previously deduced on the basis of the Planck’s vibrators, Kamerlingh Onnes was able to theoretically predict that the resistance of mercury should be lower at helium temperatures than at hydrogen temperatures and still dependent of temperature. These predictions joined to what could be considered the main expectation, which was the resistance should become, within the limits of the experimental accuracy, zero at those low temperatures [76]. After some preliminary trials that included very few experiments [84], the definitive experiences were carried out on April 8, 1911. The equipment had been again improved; the helium liquefier, which had been expanded to enclose a platinum resistor, could be now separated from the also new cryostat by closing a valve, and a double-walled vacuum pumped glass siphon cooled by a flow of liquid air replaced the old tube for liquid helium transfer. Figures 12 and 13 show a schematic representation of the whole cryostat and details of the mercury resistances, respectively. Figure 13a shows a portion of the leads of the mercury resistance partially filled with purified liquid mercury. It was constituted by seven U-shaped tubes of about 0.005 mm2 cross section connected in series, joined together by inverted Y-pieces sealed off above, and with platinum wires on both ends. Two leading tubes filled with mercury, Hg 10 and Hg 40 , through which the current entered and left, were attached to the connectors in the extremes (b0 and b8 -the last one not represented in the original figure). At solidifying, this mercury formed four leads of solid material. These leading tubes, as well as those represented Hg 20 , Hg 30 could be used for measuring the potential difference between the ends of the thread. The lead Hg 50 was left as alternative for measure the potential at b4 in order to evaluate possible variations of resistance along the full length of the thread. Figure 13b shows the manner how the tubes were really assembled inside the cryostat with the purpose of saving space for the stirring pump Sb . Figures 13c and 13d show the upper (in perspective) and front views of the resistance as it was placed inside the cryostat, respectively. Figure 13e shows other type of leads of mercury resistance, but with W-shape, used for the extension of the experiments in 1912 for measuring the resistance in four separate segments instead two. The report of the series of measurements carried out confirmed the predictions, as it can be read (the original notation is preserved): “The value of the mercury resistance used was 172.7 Ω in the liquid condition at 0 ◦ C; extrapolation from the melting point to 0 ◦ C by means of the temperature coefficient of solid mercury gives a resistance corresponding to this of 39.7 Ω in the solid state. At 4.3 K this had sunk to 0.084 Ω that is, to 0.0021 times the resistance which the solid mercury would have at 0 ◦ C. At 3 K the resistance was found to have fallen below 3 x 10−6 Ω that is to one tenmillionth of the value which it would have at 0 ◦ C.” Reif-Acherman [85]. Figura 12 - Schematic view of the cryostat used for experiments with mercury in 1911 [83]. Because the abrupt fall in the resistance, not predicted by his theoretical formula, between the melting point of hydrogen and the boiling point of helium observed in the measurements with mercury, especially between 4.21 K and 4.19 K, Kamerlingh Onnes got quickly involved in a new series of experiments in a narrower range of temperatures, in order to obtain more accurate potential measurements, and “establish beyond all possibility of doubt” that the resistance became practically zero. The main hypothesis was focused on the probable existence of an inflection point in this range of the curve representing the resistance as a function of temperature. The new experiences, which began six weeks later on May 23, and extended to October, not only confirmed the previous conclusions but also allowed an explicit reference to the jump in resis- Liquefaction of gases and discovery of superconductivity: two very closely scientific achievements in low temperature physics tivity. The results communicated at the Meeting of the Academy on November 25 and published the following month indicated that while the decreasing of the resistance was gradual between 4.29 K and 4.21 K, it was significantly rapid between 4.21 K and 4.19 K, disappearing at this latter value. Figura 13 - Details of the mercury resistance and leads [87, 88]. Figura 14 - Historical graph showing the jump in absolute resistance (Ω) of mercury in the vicinity of 4.2 K [87]. 2601-13 According to an entry in the Kamerlingh Onnes’s notebook, the experiences included the study in reverse direction; it means from lower to higher temperatures: “At 4.0 [K] not yet anything to notice of rising resistance. At 4.05 [K] not yet either. At 4.12 [K] resistance begins to appear” [75]. The details associated with this procedure recently became the subject of a sort of polemic created about the possibility that the discovery could to have been similarly to helium liquefaction the consequence of an accidental inattention and not of a programmed step in the experimental program. An anecdote of second hand told by an older visitor of the laboratory at Leiden indicate that the student of the School for Instrument Makers in charge of the differential oil manometer used to control the vapor pressure inside the cryostat was nodding off and the pressure started so to increase from below the boiling point of helium to about 4.2 K. As the transition temperature of mercury was in the route, it was obvious that a sudden movement of the light beam of the galvanometer must to have happened, showing then the restoration of the electrical resistance of the metal [86]. In the author’s opinion there is not enough primary information to support or not this historical possibility. The report of December 1911 included the historically known graph of resistance (now in absolute ohms) as a function of temperature [87]. It seems that the use of absolute resistance instead the, until then, usual ratios, was due to the uncertainty in the extrapolation to the resistance of solid mercury at 0 ◦ C from its value at the melting point. Kamerlingh Onnes named initially the new phenomenon “supraconduction” to difference the conduction process of the variable conductivity which represented the conductance of a determined material. It was clear that some “special phenomena” related with the unexpected behavior of mercury at those extreme temperatures were still obscure and that the studies should continue, as it effectively happened. Kamerlingh Onnes published several papers in the following years related with the new phenomenon. They were related with different concepts, such as critical current density and the associated potential difference, which, with the course of time, would become one of the most important for practical applications, the influence on them of different variables, as well the superconducting nature of other metals such as tin and lead [88-90]. Some of the several successive events that followed the discovery arouse curiosity and confusion. He was, for example, surprised to find that after so much care he put for purifying mercury, the simple adding of gold and cadmium to the same element did not stop it from entering the superconducting state. On the other hand, he was strongly disappointed within a couple years of his discovery; he found that a supercurrent could to be destroyed by even a small magnetic field. Some of his developments, such as the persistence over a long pe- 2601-14 riod of time of a current created in a close circuit made with the new superconducting materials even after the corresponding battery had been removed, suggest him only very few of the almost unlimited number of technological applications [91] that would begin to became reality more than three quarters of a century later [92]. 9. Concluding remarks Liquefaction of helium opened a new subject of study of low temperature physics. At referring to great transformations of his original idea at undertake his research at low temperatures in an address delivered in 1922 before the Faraday Society and the British Cold Storage and Ice Association, Kamerlingh Onnes stated that “the extension and importance which the work in this direction has attained, has widely surpassed any anticipation of mine” [93]. Indeed it was so. Many questions about the constitution of matter and the universe have been answered by the study of the phenomena at the temperatures made possible by liquid helium. As it can be said that liquefaction was the triumph of a systematic and very careful planned work, superconductivity was the unexpected finding of a program of research with almost all predictable results. The discovery of superconductivity, as well superfluidity and other events that came later, were the beginning of a torrent of technical developments that very quickly covered multiple areas. Several centers of investigation throughout the world quickly included superconductivity as a central subject of research [94]. A contemporary review on the state of the art of the subject, six decades after the discovery, reflects well the real development [95]. Semiconductor devices, superconducting magnets, magnetic resonance imaging, rocket fuels, cooling of nuclear particles, storage of biological materials, infrared sensors for target location and guidance in anti-satellite rockets, are only few of the great number of application examples of liquid helium and of technical improvements that only few decades ago began to change the style of life of the society [96]. The discovery of the high temperature superconductivity in 1986, which stimulated what has been called the revolution of these kinds of materials, decisively contributed to settle even more the striking character of the new subject [97]. In those times the new phenomenon could not be, nevertheless, theoretically understood, and its practical usefulness was considered null. Kamerlingh Onnes himself made a weak attempt for supply a theoretical explanation for superconductivity based on the supposition that the new established phenomena might be of quantum nature, according to the Einstein heat capacity theory. Quantization, as he referred in the Nobel Lecture, had introduced in physics a possibility for contribute in a fundamental way to the solution of problems related with several investigations into properties Reif-Acherman of substances at low temperature. Although his trial for contributing theoretically to the solution was unsuccessful, very probably because the difficulty to divorce himself so late in his career from classical concepts, he was more fortunate in predicting that his discovery could contribute someday for that wires carry electricity to consumers, providing so a cheap and almost unlimited supply of electricity [98]. For decades no one was able to explain how the effect worked, at such extreme that some author refers to it as “the shame and despair of theoretical physics” [99]. The German physicist Fritz London, who with his brother Heinz was the first to recognize superconductivity and superfluidity as resulting from manifestations of quantum phenomena on the scale of large objects, was clear about how classic physics represented a prejudicial attitude that hindered the development of an appropriate theory. At propose the phenomenological first theory of the electrodynamics of a superconductor, he concluded: “The present historical situation may be characterized in such a way that it is rigorously demonstrated that, on the basis of the recognized conceptions of the electron metals, a theory of superconductivity is impossible - provided that the phenomenon is interpreted in the usual way (i.e. as a kind of limiting case of ordinary conductivity) [100]. It was only in 1957, more than four and half decades later and after several constructive developments [101], when the American physicists John Bardeen (1908-1991), Leon Neil Cooper (1930-) and John Robert Schrieffer (1931-) provided an appropriate microscopic description (BCS theory) of the new phenomena [102]. Although the exact justifying words by which the Nobel Committee granted Kamerlingh Onnes the Nobel Prize in Physics in Stockholm on December 19, 1913 (“for his investigations on the properties of matter at low temperatures which led, inter alia, to the production of liquid helium”) don’t made explicit reference to superconductivity, he will be ever remembered in the annals of the history of physics as the responsible of what is considered one of the more dramatic instances in the history of low-temperature physics and to which Martin and B. Ruhemann refer in their book Low Temperature Physics as “the kind of phenomenon that every physicist would like to have discovered” [103]. Acknowledgments I thank the Museum Boerhaave for providing permission to reproduce several figures in the text and Mr. Harja Blok, Editor in chief of Refrigeration and Climate Control / Koude & Luchtbehandeling, for supply the photograph of the commemorative stone for the liquefaction of helium in my paper. I also thank the Friends of the Center for History of Physics and the Emilio Segrè Visual Archives of the American Institute of Physics, the Kamerlingh Onnes Laboratory Liquefaction of gases and discovery of superconductivity: two very closely scientific achievements in low temperature physics and the Nobel Foundation for their kind permission to use the painting of Gilles Holst, the photograph of Heike Kamerlingh Onnes, and the diagram of the cascade process, respectively. 2601-15 [11] J. Dewar, Proceedings of the Chemical Society 13, 186 (1897), reprinted in G.D. Liveing, and J. Dewar, Collected Papers on Spectroscopy (Cambridge University Press, London, 1915), p. 473-476. [12] S. Reif-Acherman, Physics in Perspective 6, 197 (2004). References [1] P.-J.-C. Janssen, Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences 67, 838 (1868). [2] E. Frankland and J.N. Lockyer, Proceedings of the Royal Society 17, 288 (1868-1869); J.N. Lockyer, Spectroscopic Observation of the Sun (Taylor and Francis, London, 1869); C.A. Russell, Edward Frankland: Chemistry, Controversy and Conspiracy in Victorian England (Cambridge University Press, Cambridge, 1996), p. 435-437. [3] T.H. Levere, in Martinus van Marum: Life and work, v. 1, edited by R.J. Forbes (Hollandsche Maatschappij der Wetenschappen, Haarlem, 1969), p. 158-286. [4] M. Faraday, Philosophical Transactions of the Royal Society of London 113, 160, 189 (1823); 135, 155 (1845); reprinted in M. Faraday, Experimental Researches in Chemistry and Physics (Taylor & Francis, London, 1991), p. 85-124. For a general accounts see W.L. Hardin, The Rise and Development of the Liquefaction of Gases (MacMillan, London, 1899), p. 569; T.O’.C. Sloane, Liquid Air and the Liquefaction of Gases (Munn & Co., New York, 1899), p. 116-151. [5] R.G. Scurlock, in History and Origins of Cryogenics, edited by R.G. Scurlock (Clarendon Press, Oxford, 1992), p. 1-47 on 6-11. See also, for example: M. Hård, Machines are Frozen Spirit: The Scientification of Refrigeration and Brewing in the 19 th Century - A Weberian Interpretation, (Campus Verlag, Frankfurt, 1994); R. Thevenot, A History of Refrigeration Throughout the World, (International Institute of Refrigeration, Paris, 1979); O.E. Anderson, Jr., Refrigeration in America: A History of a New Technology and its Impact (Princeton University Press, Princeton, 1953); E.M. Sigsworth, The Economic History Review 17, 536 (1965). [6] P.F. Dahl, Superconductivity: Its Historical Roots and Development from Mercury to the Ceramic Oxides (American Institute of Physics, New York, 1992), p. 13-22. [7] L.P. Cailletet, Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences 85, 1213 (1877). [8] R. Pictet, Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences 85, 1214 (1877); Memoire sur la Liquéfaction de l’ Oxygène et la Solidification de l’Hydrogène et sur les Theories des Changements d’état des Corps (Sandoz, Paris, 1878). [9] K. Gavroglu, European Journal of Physics 15, 9 (1994). [10] J. Dewar, Proceedings of the Royal Society of London 63, 256 (1898); partially reprinted in Collected papers of Sir James Dewar, v. 2, edited by Lady Dewar (Cambridge University Press, London, 1927), p. 678-691. [13] H. Kamerlingh Onnes, Communications Physical Laboratory University of Leiden Supplement 9, 3 (1904), on 3, reprinted in Through Measurement to Knowledge: The Selected Papers of Heike Kamerlingh Onnes 1853-1926, edited by K. Gavroglu, and Y. Goudarolis (Kluwer, Dordrecht, 1991), p. 31-58, on 31. [14] J.D. van der Waals, Over the continuiteit van den gas- en vloeistoestand, (A.W. Sijthoff, Leiden, 1873); reprinted as J.D. van der Waals, On the Continuity of the Gaseous and Liquid States (Dover, Mineola, 2004). [15] H. Kamerlingh Onnes, Communications Physical Laboratory University of Leiden 14, 3 (1894), on 4; reprinted in Through Measurement to Knowledge: The Selected Papers of Heike Kamerlingh Onnes 1853-1926, edited by K. Gavroglu, and Y. Goudarolis (Kluwer, Dordrecht, 1991), p. 3-30, on 4. [16] J.D. van der Waals, Die continuität des gasförmigen und flüssigen zustandes, translated by F. Roth (J.A. Barth, Leipzig, 1881). [17] H. Kamerlingh Onnes, Communications Physical Laboratory University of Leiden 71, 3 (1901), reprinted in Through Measurement to Knowledge: The Selected papers of Heike Kamerlingh Onnes 1853-1926, edited by K. Gavroglu, and Y. Goudarolis (Kluwer, Dordrecht, 1991), p. 146-163. [18] A. van Helden, The Coldest Spot on Earth: Kamerlingh Onnes and Low Temperature Research, 1882-1923 (Museum Boerhaave, Leiden, 1989), p. 15-25. [19] R. de Bruyn Ouboter, in Through Measurement to Knowledge: The Selected Papers of Heike Kamerlingh Onnes 1853-1926, edited by K. Gavroglu, and Y. Goudarolis (Kluwer, Dordrecht, 1991), p. xcvii-cxv, especially c-cii. [20] W.F. Hillebrand, Bulletin of the U.S. Geological Survey 78, 43 (1890). [21] M.W. Travers, Nature 135, 619 (1935). [22] P.T. Cleve, Chemical News 71, 212, 283 (1895); Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences 120, 834, 1212 (1895). [23] H.P. Cady and D.F. McFarland, Science 24, 344 (1906); Journal of the American Chemical Society 29, 1523 (1907). [24] K. Olszewski, Nature 54, 377 (1896). [25] K. Olszewski, Annalen der Physik 17, 994 (1905); Annales de Chimie et de Physique 8, 139 (1906). [26] J. Dewar, Proceedings of the Royal Society of London 68, 360 (1901), on 364-365; reprinted in Collected papers of Sir James Dewar, v. 2, edited by Lady Dewar (Cambridge University Press, London, 1927), p. 731737, on 733-36. [27] M.W. Travers, Proceedings of the Physical Society of London 17, 561 (1899). 2601-16 [28] M.W. Travers, G. Senter, G. and A. Jaquerod, Philosophical Transactions of the Royal Society of London A 200, 105 (1903). [29] M.W. Travers, Proceedings of the Royal Society of London 60, 449 (1896). [30] C.E.H. Bawn, Biographical Memoirs of the Fellows of the Royal Society 9, 300 (1963). [31] Letter from H. Kamerlingh Onnes to J. Dewar, December 29, 1903, Archives of Heike Kamerlingh Onnes at the Boerhaave Museum in Leiden, n. 294. [32] K. Gavroglu and Y. Goudaroulis, in Through Measurement to Knowledge: The Selected Papers of Heike Kamerlingh Onnes 1853-1926, edited by K. Gavroglu, and Y. Goudarolis (Kluwer, Dordrecht, 1991), p. xiiixcvi. [33] Letter from J.Dewar to H. Kamerlingh Onnes, June 8, 1905. Reif-Acherman [46] H. Davy, Philosophical Transactions of the Royal Society of London, 111, 425 (1821). [47] E. Lenz, Annalen der Physik (Leipzig) 34, 418 (1835); Annalen der Physik (Leipzig) 45, 105 (1838). [48] A.F.O. Arndtsen, Annalen der Physik (Leipzig) 104, 1 (1858); Annales de Chimie et de Physique 54, 440 (1858). [49] R. Clausius, Annalen der Physik (Leipzig) 104, 650 (1858). [50] Matthiessen, Annalen der Physik (Leipzig) 103, 428 (1858); Philosophical Transactions of the Royal Society of London 148, 383 (1858). [51] L.P. Cailletet and E.M.L. Bouty, Journal de Physique Théorique et Appliquée 4, 297 (1885); Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences 100, 1187 (1885); Philosophical Magazine 20, 77 (1885). [34] D.O. Wood, Proceedings of the Royal Society of London, Series A, 84, 70 (1910); M.W. Travers, Proceedings of the Royal Society of London, 64, 130 (18981899). [52] A. Righi, Journal de Physique Théorique et Appliquée 3, 355 (1884). [35] H. Kamerlingh Onnes, Communications Physical Laboratory University of Leiden 108, 3 (1908); reprinted in Through Measurement to Knowledge: The Selected Papers of Heike Kamerlingh Onnes 1853-1926, edited by K. Gavroglu, and Y. Goudarolis (Kluwer, Dordrecht, 1991), p. 164-187. [54] E. van Aubel, Philosophical Magazine 28, 332 (1889). [36] R. de Rruyn Ouboter, Institute of Electrical and Electronics Engineers Transactions on Magnetics MAG23, 355 (1987); D. van Delft, Physics Today 61, 36 (2008). [56] Z. Wróblewski, Annalen der Physik (Leipzig) 26, 27 (1885); Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences 101, 160 (1885). [37] H. Kamerlingh Onnes, in Nobel Lectures Including Presentation Speeches and Laureates’s Biographies, Physics 1901-1921, v. 1, edited by Nobel Foundation, (Elsevier, Amsterdam, 1967). [38] J. Matricon and G. Waysand, La Guerre du Froid: Une Histoire de la Supraconductivité (Édtions du Seuil, Paris, 1994), p. 48. [39] K. Gavroglu and Y. Goudaroulis, Methodological Aspects of the Development of Low-Temperature Physics 1881-1956 (Kluwer Academic Press, Dordrecht, 1989), p. 62-70. [40] A. Laesecke, Journal of Research of the National Institute of Standards and Technology 107, 261 (2002). [41] B. Meilink, Proceedings of the Royal Netherlands Academy of Arts and Sciences 4, 495 (1901-1902). [42] B. Meilink, Proceedings of the Royal Netherlands Academy of Arts and Sciences 7, 290 (1904-1905). [53] A. Leduc, Journal de Physique Théorique et Appliquée 3, 362 (1884). [55] T. Estreicher, in Great Men and Women of Poland, edited by S.P. Mizwa (MacMillan, New York, 1942), p. 263-277; J. Rafalowicz, in: History and Origins of Cryogenics, edited by R.G. Scurlock (Clarendon Press, Oxford, 1992), p. 101-111. [57] H.L. Callendar, Philosophical Magazine 48, 519 (1899). [58] J. Dewar and J.A. Fleming, Philosophical Magazine 34, 326 (1892), reprinted in Collected Papers of Sir James Dewar, v. 1, edited by Lady Dewar (Cambridge University Press, London, 1927), p. 339-348. [59] J. Dewar and J.A. Fleming, Philosophical Magazine 36, 271 (1893); reprinted in Collected Papers of Sir James Dewar, v. 1, edited by Lady Dewar (Cambridge University Press, London, 1927), p. 363-389. [60] http://www.inghist.nl/Onderzoek/Projecten/BWN/ lemmata/bwn1/claij, accessed in October, 2010. [61] H. Kamerlingh Onnes, and J. Clay, Proceedings of the Royal Netherlands Academy of Arts and Sciences 9, 207 (1906). [62] H. Kamerlingh Onnes and J. Clay, Proceedings of the Royal Netherlands Academy of Arts and Sciences 9, 213 (1906). [43] B. Meilink, Proceedings of the Royal Netherlands Academy of Arts and Sciences 7, 300 (1904-1905). [63] J. Clay and H. Kamerlingh Onnes, Proceedings of the Royal Netherlands Academy of Arts and Sciences 10, 207 (1907). [44] J.L. Heilbron, Electricity in the 17 th and 18 th Centuries: A Study in Early Modern Physics (Dover Publications, Mineola, 1979), p. 368. [64] J. Clay and H. Kamerlingh Onnes, Proceedings of the Royal Netherlands Academy of Arts and Sciences 10, 342 (1907). [45] H. Cavendish, Philosophical Transactions of the Royal Society of London 61, 584 (1771); Philosophical Transactions of the Royal Society of London 66, 196 (1776). [65] J. Clay and H. Kamerlingh Onnes Proceedings of the Royal Netherlands Academy of Arts and Sciences 10, 200 (1907). Liquefaction of gases and discovery of superconductivity: two very closely scientific achievements in low temperature physics [66] J. Dewar, Proceedings of the Royal Society 68, 360 (1901), reprinted in Lady Dewar (Ed.), Collected Papers of Sir James Dewar, v. 2 (Cambridge University Press, Cambridge 1927), p.731-737. [67] J. Dewar, Proceedings of the Royal Society of London 73, 244 (1904), reprinted in Collected Papers of Sir James Dewar, v. 2, edited by Lady Dewar (Cambridge University Press, London, 1927), p. 847-855. [68] M.W. Travers and A.G.C. Gwyer, Proceedings of the Royal Society of London 74, 528 (1904-1905). [69] E. Riecke, Annalen der Physik (Leipzig) 66, 353 (1898); Annalen der Physik (Leipzig) 2, 835 (1900). [70] P. Drude, Annalen der Physik (Leipzig) 1, 566 (1900). [71] H.A. Lorentz, Proceedings of the Royal Netherlands Academy of Arts and Sciences 7, 438 (1904-1905); Proceedings of the Royal Netherlands Academy of Arts and Sciences 7, 585 (1904-1905); Proceedings of the Royal Netherlands Academy of Arts and Sciences 7, 684 (1904-1905). [72] A. Matthiessen and C. Vogt, Philosophical Transactions of the Royal Society of London 154, 167 (1864). [73] Lord Kelvin, Philosophical Magazine 3, 257 (1902), reprinted in Baltimore Lectures, 1904, Appendix E, p. 541-568. [74] Lord Kelvin, Archives Néerlandaises des Sciences Exactes et Naturelles 6, 834 (1901). [75] D. van Delft and P. Kes, Physics Today 63, 38 (2010). [76] H. Kamerlingh Onnes, Proceedings of the Royal Netherlands Academy of Arts and Sciences 13, 1107 (1910-1911). [77] H.B.G. Casimir, G. Holst : Pionier van industriel onderzoek in Nederland (Holst-Lezing - Technische Hogeschool Eindhoven, Eindhoven (1981); Haphazard reality: Half a century of science (Harper Row Publishers, New York, 1983), p. 165-166, p. 230238; http://www.inghist.nl/Onderzoek/Projecten/ BWN/lemmata/bwn1/holst, accessed in October, 2010. [78] P.F. Dahl, Historical Studies in the Physical Sciences 15, 1 (1984). [79] M. Planck, Verhandlungen der Deutsche Physikalische Gesellschaft 13, 138 (1911). [80] W. Nernst, Nachrichten von der Königl. Gesellschaft der Wissenschaften und der Georg-Augusts-Universität zu Göttingen 1, 1 (1906); Berliner Berichte 1, 247, 262, 306 (1910). See also D.K. Barkan, in World Views and Scientific Discipline Formation, edited by W.R. Woodward (Kluwer, Dordrecht, 1991), p. 151-162. [81] A. Einstein, Annalen der Physik 22, 180 (1907). [82] D.K. Barkan, Walther Nernst and the Transition to Modern Physical Science (Cambridge University Press, Cambridge, 1999), p. 161. [83] H. Kamerlingh Onnes, Proceedings of the Royal Netherlands Academy of Arts and Sciences 14, 204 (1911). 2601-17 [84] H. Kamerlingh Onnes, Proceedings of the Royal Netherlands Academy of Arts and Sciences 13, 1274 (1910-1911). [85] H. Kamerlingh Onnes, Proceedings of the Royal Netherlands Academy of Arts and Sciences 14, 113 (1911). [86] J. de Nobel, Physics Today 49, 40 (1996). [87] H. Kamerlingh Onnes, Proceedings of the Royal Netherlands Academy of Arts and Sciences 14, 818 (1911-1912). [88] H. Kamerlingh Onnes, Proceedings of the Royal Netherlands Academy of Arts and Sciences 15, 1406 (1912-1913). [89] H. Kamerlingh Onnes, Proceedings of the Royal Netherlands Academy of Arts and Sciences 16, 113 (1913). [90] H. Kamerlingh Onnes, Proceedings of the Royal Netherlands Academy of Arts and Sciences 16, 673 (1913-1914). [91] K. Gavroglu and Y. Goudaroulis, Studies in History and Philosophy of Science 19, 243 (1988). [92] T.H. Geballe, Physics Today 46, 52 (1993). [93] H. Kamerlingh Onnes, in Kostas Gavroglu and Yorgos Goudaroulis, ed., Through Measurement to Knowledge: The Selected Papers of Heike Kamerlingh Onnes 18531926 (Kluwer, Dordrecht, 1991), p. 59-88, on 60. [94] A.B. Pippard, Institute of Electrical and Electronics Engineers Transactions on Magnetics MAG-23, 371 (1987); C.G. Gorter, Reviews of Modern Physics 36, 3 (1964); K. Mendelssohn, Reviews of Modern Physics 36, 7 (1964); P.F. Dahl, Historical Studies in the Physical Sciences 16, 1 (1986). [95] J. Bardeen in Studies in the Natural Sciences. I. Impact of Basic Research in Technology, edited by B. Kursunoglu and A. Perlmutter (Plenum Press, New York, 1973), p. 1-57. [96] E.T. Smith, Business Week 2991, 54 (1987); J.W. Wilson, Business Week 2991, 58 (1987), M.D. Lemonick, Science 129, 26 (1987). [97] T.H. Geballe and J.K. Hulm, Science 239, 367 (1988). [98] R. de Bruyn Ouboter, Scientific American 276, 98 (1997). [99] J.M. Blatt, Theory of Superconductivity (Academic Press, New York, 1964), p. V. [100] F. London, Proceedings of the Royal Society of London 152, 24 (1935). [101] J. Bardeen, Physics Today 1, 21 (1963). [102] J. Bardeen, L.N. Cooper and J.R. Schrieffer, Physical Review 108, 1175 (1957). [103] M. Ruhemann and B. Ruhemann, Low Temperature Physics (Cambridge University Press, Cambridge, 1937), p. 270.