Determination of Molar Mass by
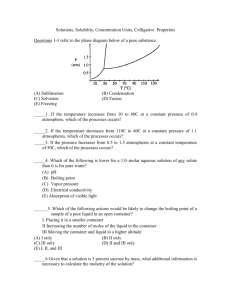
Freezing-Point Depression
When a solute is dissolved in a solvent, the freezing temperature is lowered in proportion to the number of
moles of solute added. This property, known as freezing-point depression, is a colligative property; that is, it
depends on the ratio of solute and solvent particles, not on the nature of the substance itself. The equation
that shows this relationship is
ΔTf = Kf × m× i
where ΔTf is the freezing point depression, Kf is the freezing point depression constant for a particular
solvent (3.9°C•kg/mol for lauric acid in this experiment1), i is the van’t Hoff factor, and m is the molality of
the solution (in mol solute/kg solvent). Since lauric acid is not ionic, its van’t Hoff factor is essentially equal
to 1.
OBJECTIVES
In this experiment, you will
•
•
•
•
Determine the freezing temperature of the pure solvent, lauric acid.
Determine the freezing temperature of a mixture of lauric acid and benzoic acid.
Calculate the freezing point depression of the mixture.
Calculate the molecular weight of benzoic acid.
Figure 1
MATERIALS
Data Collection Mechanism
Temperature Probe
ring stand
400 mL beaker
Tissue or paper towels
lauric acid, CH3(CH2)10COOH
lauric acid-benzoic acid mixture
hot water bath
utility clamp
two 18 × 150 mm test tubes (if pre-made samples
are not provided by your teacher)
1
“The Computer-Based Laboratory”, Journal of Chemical Education: Software, 1988, Vol.1A, No. 2, p. 73.
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
The Determination of Molar Mass by Freezing-Point Depression
PROCEDURE
1. Obtain and wear goggles.
2. Set up the data collection system.
a. Connect a Temperature Probe to the interface.
b. Start the data collection program.
c. Set up the time graph for 10 seconds per sample and 60 samples.
Part I: Determine the Freezing Temperature of Pure Lauric Acid
3. Add about 300 mL of tap water with a temperature of 20-25°C to a 400 mL beaker. Place the beaker on
the base of the ring stand.
4. Use a utility clamp to obtain a test tube containing hot, melted lauric acid from your instructor. Fasten the
utility clamp at the top of the test tube. CAUTION: Be careful not to spill the hot lauric acid on yourself
and do not touch the bottom of the test tube.
5. Insert the Temperature Probe into the hot lauric acid. Fasten the utility clamp to the ring stand so the test
tube is above the water bath.
6. Begin data collection. Lower the test tube into the water bath. Make sure the water level outside the test
tube is higher than the lauric acid level in the test tube, as shown in Figure 1.
7. With a very slight up-and-down motion of the Temperature Probe, continuously stir the lauric acid for the
ten-minute duration of the experiment. Do not allow the temperature probe to touch the bottom of the
test tube.
8. When data collection is complete, use a hot water bath to melt the lauric acid enough to safely remove
the Temperature Probe. Carefully wipe any excess lauric acid liquid from the probe with a paper towel or
tissue.
9. The freezing temperature can be determined by finding the mean temperature in the portion of the graph
with nearly constant temperature.
a. Select the data in the flat region of the graph.
b. Find the mean temperature for the selected data. Record this value.
c. Store the data, so that they can be used later.
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
The Determination of Molar Mass by Freezing-Point Depression
Part II: Determine the Freezing Point of a Solution of Benzoic Acid and Lauric Acid
10. Obtain a test tube containing a melted solution with ~1 g of benzoic acid dissolved in ~8 g of lauric acid.
Record the precise masses of benzoic acid and lauric acid as indicated on the label of the test tube.
Freezing Point
Time
Repeat Steps 3-8.
11. The freezing point of the benzoic acid-lauric acid solution can be determined by finding the temperature
at which the mixture initially started to freeze. Unlike pure lauric acid, the mixture results in a gradual
linear decrease in temperature during freezing.
12. Print a graph showing both trials. (See the graph pictured in question 6 of the Pre-Lab Questions.)
DATA TABLE
Mass of lauric acid in the benzoic acid-lauric acid mixture (g)
Mass of benzoic acid in the benzoic acid-lauric acid mixture (g)
Freezing temperature of pure lauric acid (°C)
Freezing point of the benzoic acid-lauric acid mixture (°C)
PRE-LAB QUESTIONS
1. What types of intermolecular forces are present in a molecular solid such as lauric acid? Describe what is
happening with regard to intermolecular forces as a molecular liquid freezes.
2. If you were able to choose a solvent for this experiment from the list below, which would you choose?
Justify your answer.
•
•
•
•
Acetic Acid CH3COOH
Benzene C6H6
tert-Butanol C4H9OH
Cyclohexane C6H12
Kf
Kf
Kf
Kf
=
=
=
=
3.90 °C•kg/mol
5.12 °C•kg/mol
9.10 °C•kg/mol
20.00 °C•kg/mol
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
The Determination of Molar Mass by Freezing-Point Depression
3. What is the equation for calculating molality? Why do we use molality rather than molarity as our
concentration unit for this experiment?
4. Use the equation above along with the freezing-point depression equation to derive an expression for
calculating the molar mass of a solute.
5. The following data were obtained in an experiment designed to determine the molar mass of a solute by
freezing-point depression. The Kf of para-dichlorobenzene is 7.1 °C•kg/mol
Mass of para-dichlorobenzene (g)
Mass of unknown solute (g)
24.80 g
2.04 g
Freezing temperature of pure para-dichlorobenzene (°C)
53.02 °C
Freezing point of the solution (°C)
50.78 °C
(a) Calculate the freezing-point depression, ΔTf of the solution.
(b) Calculate the molar mass of the unknown substance.
6. Examine the graph below. What laboratory technique best prevents supercooling?
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
The Determination of Molar Mass by Freezing-Point Depression
POST-LAB QUESTIONS AND DATA ANALYSIS
1. Calculate molality (m), in mol/kg, using the formula ΔTf = Kf × m × i. The Kf value for lauric acid is
3.9°C•kg/mol and since lauric acid is a molecular solid, i is approximately equal to 1.
2. Calculate moles of benzoic acid solute, using the molality and the mass (in kg) of lauric acid solvent.
3. Calculate the experimental molecular weight of benzoic acid, in g/mol.
4. Determine the accepted molecular weight of benzoic acid from its formula, C6H5COOH.
5. Calculate the percent error between the experimental and accepted values.
6. Explain why the pure solvent shows a level horizontal curve as solidification occurs, but the curve for
the solution slopes downward slightly.
7. A student spills some of the solvent before the solute was added. What effect does this error have on
the calculated molar mass of the solute? Mathematically justify your answer.
8. A different student spills some of the solution before the freezing-point was determined. What effect
does this error have on the calculated molar mass of the solution? Justify your answer.
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
The Determination of Molar Mass by Freezing-Point Depression
TEACHER INFORMATION
1. This experiment conforms to the guidelines for the fourth laboratory experiment listed in the
College Board AP Chemistry guide (the Acorn book).
2. Lauric acid, CH3(CH2)10COOH, has an accepted melting point of 44.0°C and a molecular mass
of 122.0 g/mol. It is also called dodecanoic acid.
3. The ΔTf value limits the number of significant figures of the final answer for molecular mass to two or
more depending on your temperature probe or thermometer. Thus, the final molecular mass in the
sample data is expressed as 120 g/mol not 119 g/mol.
4. Test tube sizes 18 × 150 mm, 20 × 150 mm, or 25 × 150 mm work well.
5. These can be prepared well in advance. For Part I, put about 8 g of lauric acid in each tube (once
you’ve done the first one, you can guess for the rest since the amount doesn’t matter). Number each
tube 1-2, 1-2, etc.. For Part II, mix about 8 grams of lauric acid with about 1 gram of benzoic acid per
test tube. Label each test tube with a number 2-1, 2-2, etc. and the precise mass of lauric acid and
benzoic acid and make sure that the label will be above the water level of the water bath. These filled
test tubes may be reused, as long as your students avoid cross-contaminating the test tubes with the
Temperature Probe. Stopper the test tubes and store them for future use. You may also make one large
“batch” of the 8g:1g ratio, but be sure to melt the mixture to ensure complete mixing and pour it into
the tubes. Each tube will need to be labeled with the precise masses of each of the two substances.
6. It is a good idea to have hot plates with water baths warming up, before students arrive, to save time.
7. Prepare a separate hot water bath in a central location for the students to use to free the probes that
have been frozen in test tubes.
HAZARD ALERTS
Lauric acid: Slightly toxic by ingestion; body tissue irritant; combustible. Hazard Code: C—Somewhat
hazardous.
Benzoic acid: Slightly toxic by ingestion; body tissue irritant; combustible. Hazard Code: C—Somewhat
hazardous.
The hazard information reference is: Flinn Scientific, Inc., Chemical and Biological Catalog Reference
Manual, P.O. Box 219, Batavia, IL 60510, (800) 452-1261, www.flinnsci.com
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
The Determination of Molar Mass by Freezing-Point Depression
SAMPLE DATA TABLE
Mass of lauric acid in the lauric acid-benzoic acid mixture (g)
8.01 g
Mass of benzoic acid in the lauric acid-benzoic acid mixture (g)
1.00 g
Freezing temperature of pure lauric acid (°C)
44.0°C
Freezing point of the benzoic acid-lauric acid mixture (°C)
39.9°C
Answers to PRE-LAB QUESTIONS
1. What types of intermolecular forces are present in a molecular solid such as lauric acid? Describe what it
happening with regard to both energy and intermolecular forces as a molecular liquid freezes.
Since lauric acid is molecular, the most prevalent IMFs are London dispersion forces (induced dipoleinduced dipole). As the sample cools, the temperature decreases hence the average kinetic energy of the
molecules decreases and the molecules slow down. At some point, enough heat is removed so that the
attractive forces of the molecules bring them closer together, and their positions become fixed and the
substance freezes.
2. If you were able to choose a solvent for this experiment from the list below, which would you choose?
Justify your answer.
•
•
•
•
Acetic Acid CH3COOH
Benzene C6H6
tert-Butanol C4H9OH
Cyclohexane C6H12
Kf
Kf
Kf
Kf
=
=
=
=
3.90 °C•kg/mol
5.12 °C•kg/mol
9.10 °C•kg/mol
20.00 °C•kg/mol
Cyclohexane. The larger the value of the freezing-point depression constant, the more precise the molar
mass can be determined considering the small values of temperature change for this type of experiment.
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
The Determination of Molar Mass by Freezing-Point Depression
3. What is the equation for calculating molality? Why do we use molality rather than molarity as our
concentration unit for this experiment?
mass solute (g)
moles solute
MM Students should receive full credit for either version
m=
=
kg solvent
kg solvent
We use molality for colligative property experiments since it is temperature independent and is
essentially a ratio of masses (solute : solvent)which does not change with temperature changes. Molarity
incorporates the volume of the solution which can expand or contract with changes in temperature.
4. Use the equation above along with the freezing-point depression equation to derive an expression for
calculating the molar mass of a solute.
mass solute (g)
moles solute
MM and ΔT = K × m × i
m=
=
f
f
kg solvent
kg solvent
g
∴ΔT f = K f × MM × i ; where i ≈ 1
kg
g
∴ΔT f (kg ) = K f
∴ΔT f (kg ) MM = K f g
MM
K f g ( solute)
∴ MM =
ΔT f (kg solvent )
5. The following data were obtained in an experiment designed to determine the molar mass of a solute by
freezing-point depression. The Kf of para-dichlorobenzene is 7.1 °C•kg/mol
Mass of para-dichlorobenzene (g)
Mass of unknown solute (g)
24.80 g
2.04 g
Freezing temperature of pure para-dichlorobenzene (°C)
53.02 °C
Freezing point of the solution (°C)
50.78 °C
(a) Calculate the freezing-point depression, ΔTf of the solution.
ΔT f = ( 53.02 − 50.78 ) °C
(b) Calculate the molar mass of the unknown substance.
Note that i ≈ 1
°C • kg
× 2.04 g
g
mol
∴ MM =
=
= 260
ΔT f (kg solvent ) 0.02480 kg × 2.24°C
mol
Note that the precision of your thermometer will dictate the number of sig. figs reported on the
molar mass.
K f g ( solute)
7.1
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
The Determination of Molar Mass by Freezing-Point Depression
6. Examine the graph below. What laboratory technique best prevents supercooling?
Stirring constantly during the cooling process.
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
The Determination of Molar Mass by Freezing-Point Depression
Answers to POST-LAB QUESTIONS AND DATA ANALYSIS
1. Calculate molality (m), in mol/kg, using the formula ΔTf = Kf × m × i. The Kf value for lauric acid is
3.9°C•kg/mol and since lauric acid is a molecular solid, i is approximately equal to 1.
ΔT f = K f × m × i; where i ≈ 1
∴m =
ΔT f
Kf
=
4.1°C
mol
= 1.05
°C • kg
kg
3.9
mol
2. Calculate moles of benzoic acid solute, using the molality and the mass (in kg) of lauric acid solvent.
Answers will vary. For the sample data,
mol
1.05
× 0.00801 kg solvent = 0.00841 mol benzoic acid
kg
3. Calculate the experimental molecular weight of benzoic acid, in g/mol.
°C • kg ⎞
⎛
3.9
⎜
⎟ (1.00 g )
K f g ( solute)
g
g
mol ⎠
⎝
∴ MM =
=
= 118.8
= 120
mol
mol
ΔT f (kg solvent ) ( 4.1°C )( 0.00801 kg )
Since the change in temperature is reported as 2 SF.
4. Determine the accepted molecular weight of benzoic acid from its formula, C6H5COOH.
The accepted molar mass is the molar mass calculated from the chemical formula given and is equal
to 122 g/mol.
5. Calculate the percent error between the experimental and accepted values.
% error =
122 − 120
122
× 100 = 0.02 % error
6. Explain why the pure solvent shows a level horizontal curve as solidification occurs, but the curve for the
solution slopes downward slightly.
A pure substance maintains a constant as it freezes. When a solution freezes, the pure solvent freezes
first. As it solidifies, the remaining solution is more concentrated so its freezing point is lower.
7. A student spills some of the solvent before the solute was added. What effect does this error have on the
calculated molar mass of the solute? Mathematically justify your answer.
The calculated molar mass will be too high. MM =
K f g ( solute)
, therefore a smaller number for kg
ΔT f (kg solvent )
of solvent in the denominator will result in a larger calculated molar mass.
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
The Determination of Molar Mass by Freezing-Point Depression
8. A different student spills some of the solution before the freezing-point was determined. What effect
does this error have on the calculated molar mass of the solution? Justify your answer.
The calculated molar mass will unaffected. The spill will not alter the ratio of solute molecules to
solvent molecules; therefore the molality of the solution remains the same before and after the spill.
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
2000 AP® CHEMISTRY FREE-RESPONSE QUESTIONS
Your responses to the rest of the questions in this part of the examination will be graded on the basis of the accuracy
and relevance of the information cited. Explanations should be clear and well organized. Examples and equations
may be included in your responses where appropriate. Specific answers are preferable to broad, diffuse responses.
Answer BOTH Question 5 below AND Question 6 printed on page 11. Both of these questions will be graded. The
Section II score weighting for these questions is 30 percent (15 percent each).
5. The molar mass of an unknown solid, which is nonvolatile and a nonelectrolyte, is to be determined by the
freezing-point depression method. The pure solvent used in the experiment freezes at 10°C and has a known
molal freezing-point depression constant, Kf . Assume that the following materials are also available.
test tubes
beaker
stirrer
stopwatch
pipet
graph paper
thermometer
hot-water bath
balance
ice
(a) Using the two sets of axes provided below, sketch cooling curves for (i) the pure solvent and for (ii) the
solution as each is cooled from 20°C to 0.0°C.
(b) Information from these graphs may be used to determine the molar mass of the unknown solid.
(i) Describe the measurements that must be made to determine the molar mass of the unknown solid by
this method.
(ii) Show the setup(s) for the calculation(s) that must be performed to determine the molar mass of the
unknown solid from the experimental data.
(iii) Explain how the difference(s) between the two graphs in part (a) can be used to obtain information
needed to calculate the molar mass of the unknown solid.
(c) Suppose that during the experiment a significant but unknown amount of solvent evaporates from the test
tube. What effect would this have on the calculated value of the molar mass of the solid (i.e., too large, too
small, or no effect)? Justify your answer.
(d) Show the setup for the calculation of the percentage error in a student’s result if the student obtains a value
of 126 g mol−1 for the molar mass of the solid when the actual value is 120. g mol−1.
Copyright © 2000 College Entrance Examination Board and Educational Testing Service. All rights reserved.
AP is a registered trademark of the College Entrance Examination Board.
GO ON TO THE NEXT PAGE.
-10Page 12
AP® Chemistry 2000 ─ Scoring Standards
Question 5
(10 points)
Pure Solvent
Solution
20
20
15
15
Temperature (oC)
Temperature (oC)
(a)
10
5
0
10
5
0
Time
2 pts.
Time
Notes: One point is earned for each correct graph. The first graph should show a
line that drops to 10°C, holds steady at 10°C, and then falls steadily to 0°C. There
must be a discernable plateau at 10°C to earn this point. The second graph should
show a line that drops to below 10°C, levels off (or slants down a bit), and then falls
more sharply to 0°C.
(b)
(i) Measure mass of solute, mass of solvent, mass of solution
(two of three must be shown)
1 pt.
Measure the ∆Tfp (or the freezing point of the solution)
1 pt.
• Volume of solution (without density), molality, or number of
moles do not earn points
(ii) Given: ∆T = iKf m (or ∆T = Kf m)
m = (mol solute)/(kg solvent)
moles = g/(molar mass)
2 pts.
Combine to get: molar mass = (i)(Kf)(g solute)/(∆T)(kg solvent)
Notes: One point is earned for any two equations, and two points are earned
for all three equations. “Solute” and “solvent” must be clearly identified in
the equations.
(iii) the difference in the vertical position of the horizontal portions of the graphs
1 pt.
is equal to ∆Tfp , the change in freezing point due to the addition of the solute.
Copyright © 2000 College Entrance Examination Board and Educational Testing Service. All rights reserved.
AP is a registered trademark of the College Entrance Examination Board.
Page 13
AP® Chemistry 2000 ─ Scoring Standards
Question 5
(continued)
(c)
The molar mass is too small.
1 pt.
If some of the solvent evaporates, then the (kg solvent) term used in the
equation in (b) (ii) is larger than the actual value. If the (kg solvent)
term used is too large, then the value calculated for the molar mass will be
too small.
1 pt.
or
If some of the solvent evaporates, then the concentration (molality) of the
solute will be greater than we think it is. More moles of solute results in a
smaller molar mass (or since ∆T = iKf m, then the ∆Tobs would be greater
than it should be). Since the molar mass of the unknown solute is inversely
proportional to ∆T, an erroneously high value for ∆T implies an erroneously
low value for the molar mass (calculated molar mass would be too small).
(d)
% error =
(126 g mol −1 − 120 g mol −1 )
120 g mol −1
× 100%
1 pt.
or
% error =
6 g mol −1
120 g mol −1
× 100%
Copyright © 2000 College Entrance Examination Board and Educational Testing Service. All rights reserved.
AP is a registered trademark of the College Entrance Examination Board.
Page 14
Page 15
Page 16