Solutions, Solubility, Concentration Units, Colligative Properties
advertisement
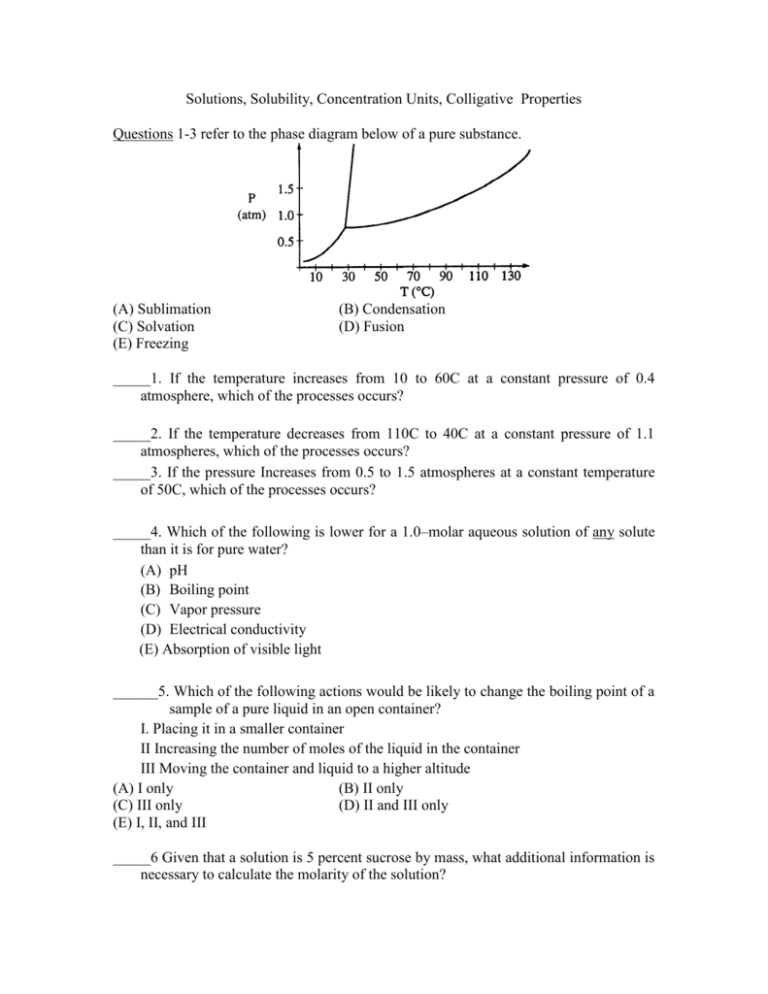
Solutions, Solubility, Concentration Units, Colligative Properties Questions 1-3 refer to the phase diagram below of a pure substance. (A) Sublimation (C) Solvation (E) Freezing (B) Condensation (D) Fusion _____1. If the temperature increases from 10 to 60C at a constant pressure of 0.4 atmosphere, which of the processes occurs? _____2. If the temperature decreases from 110C to 40C at a constant pressure of 1.1 atmospheres, which of the processes occurs? _____3. If the pressure Increases from 0.5 to 1.5 atmospheres at a constant temperature of 50C, which of the processes occurs? _____4. Which of the following is lower for a 1.0–molar aqueous solution of any solute than it is for pure water? (A) pH (B) Boiling point (C) Vapor pressure (D) Electrical conductivity (E) Absorption of visible light ______5. Which of the following actions would be likely to change the boiling point of a sample of a pure liquid in an open container? I. Placing it in a smaller container II Increasing the number of moles of the liquid in the container III Moving the container and liquid to a higher altitude (A) I only (B) II only (C) III only (D) II and III only (E) I, II, and III _____6 Given that a solution is 5 percent sucrose by mass, what additional information is necessary to calculate the molarity of the solution? I. II. III. (A) (D) The density of water The density of the solution The molar mass of sucrose I only (B) II only (C) III only I and III (E) II and III _____7 If 87 grams of K2SO4 (molar mass 174 grams) is dissolved in enough water to make 250 milliliters of solution, what are the concentrations of the potassium and the sulfate ions? [K+] [SO42–] (A) 0.020 M 0.020 M (B) 1.0 M 2.0 M (C) 2.0 M 1 .0 M (D) 2.0 M 2.0 M (E) 4.0 M 2.0 M _____8. When a 1.00–gram sample of limestone was dissolved in acid, 0.38 gram of CO2 was generated. If the rock contained no carbonate other than CaCO3, what was the percent of CaCO3 by mass in the limestone? (A) 17% (B) 51% (C) 64% (D) 86% (E) 100% _____9 It is suggested that SO2 (molar mass = 64.1 grams), which contributes to acid rain, could be removed from a stream of waste gases by bubbling the gases through 0.25–molar KOH, thereby producing K2SO3. What is the maximum mass of SO2 that could be removed by 1,000. liters of the KOH solution? (A) 4.0 kg (B) 8.0 kg (C) 16 kg (D) 20. kg (E) 40. kg _____10. What mass of Au is produced when 0.0500 mol of Au2S3 is reduced completely with excess H2 ? (A) 9.85 g (B) 19.7 g (C) 24.5 g (D) 39.4 g (E) 48.9 g _____11 The cooling curve for a pure substance as it changes from a liquid to a solid is shown above. The solid and the liquid coexist at (A) point Q only (B) point R only (C) all points on the curve between Q and S (D) all points on the curve between R and T (E) no point on the curve _____12. If the temperature of an aqueous solution of NaCl is increased from 20C to 90C which of the following statements is true? (A) The density of the solution remains unchanged. (B) The molarity of the solution remains unchanged. (C) The molality of the solution remains unchanged. (D) The mole fraction of solute decreases. (E) The mole fraction of solute increases. _____13. A 1.0 L sample of an aqueous solution contains 0.10 mol of NaCl and 0.10 mol of CaCl2. What is the minimum number of moles of AgNO3 that must be added to the solution in order to precipitate all of the Cl– as AgCl(s) ? (Assume that AgCl is insoluble.) (A) 0.10 mol (B) 0.20 mol (C) 0.30 mol (D) 0.40 mol (E) 0.60 mol _____14 On a mountaintop, it is observed that water boils at 90C not at 100C as at sea level. This phenomenon occurs because on the mountaintop the (A) equilibrium water vapor pressure is higher due to the higher atmospheric pressure (B) equilibrium water vapor pressure is lower due to the higher atmospheric pressure (C) equilibrium water vapor pressure equals the atmospheric pressure at a lower temperature (D) water molecules have a higher average kinetic energy due to the lower atmospheric pressure (E) water contains a greater concentration of dissolved gases _____15 A 40.0 mL sample of 0.25 M KOH is added to 60.0 mL of 0.15 M Ba(OH)2. What is the molar concentration of OH–(aq) in the resulting solution? (Assume that the volumes are additive.) (A) 0.10 M (B) 0.19 M (C) 0.28 M (D) 0.40 M (E) 0.55 M _____16 What is the final concentration of barium ions, [Ba2+], in solution when 100. mL of 0.10 M BaCl2(aq) is mixed with 100. mL of 0.050 M H2SO4(aq)? (A) 0.00 M (B) 0.012 M (C) 0.025 M (D) 0.075 M (E) 0.10 M _____17. When 100 mL of 1.0 M Na3PO4 is mixed with 100 mL of 1.0 M AgNO3, a yellow precipitate forms and [Ag+] becomes negligibly small. Which of the following is a correct listing of the ions remaining in solution in order of increasing concentration? (A) [PO43–] < [NO3–] < [Na+] (B) [PO43–] < [Na+] < [NO3–] (C) [NO3–] < [PO43–] < [Na+] (D) [Na+] < [NO3–] < [PO43–] (E) [Na+] < [PO43–] < [NO3–] _____18 In a qualitative analysis for the presence of Pb2+, Fe2+, and Cu2+ ions in aqueous solution, which of the following will allow the separation of Pb2+ from the other ions at room temperature? (A) Adding dilute Na2S(aq) solution (B) Adding dilute HCl(aq) solution (C) Adding dilute NaOH(aq) solution (D) Adding dilute NH3(aq) solution (E) Adding dilute HNO3(aq) solution _____19. The volume of distilled water that should be added to 10.0 mL of 6.00 M HCl(aq) in order to prepare a 0.500 M HCl(aq) solution is approximately (A) 50.0 mL (B) 60.0 mL (C) 100. mL (D) 110. mL (E) 120. mL _____20 The weight of H2SO4 (molecular weight 98.1) in 50.0 milliliters of a 6.00-molar solution is a. 3.10 grams c. 29.4 grams e. 300. grams b. 12.0 grams d. 294 grams 21. Which of the following is true at the triple point of a pure substance? a. The vapor pressure of the solid phase always equal the vapor pressure of the liquid phase. b. The temperature is always 0.01 K lower that the normal melting point. c. The liquid and gas phases of the substance always have the same density and are therefore indistinguishable. d. The solid phase always melts if the pressure increases at constant temperature. e. The liquid phase always vaporizes if the pressure increases at constant temperature. 22 I. Difference in temperature between freezing point of solvent and freezing point of solvent and freezing point of solution II. Molal freezing point depression constant, Kƒ, for solvent In addition to the information above, which of the following gives the minimum data required to determine the molecular mass of a nonionic substance by the freezing point depression technique? a. No further information is necessary. b. Mass of solute c. Mass of solute and mass of solvent d. Mass of solute and volume of solvent e. Mass of solute, mass of solvent, and vapor pressure of solvent