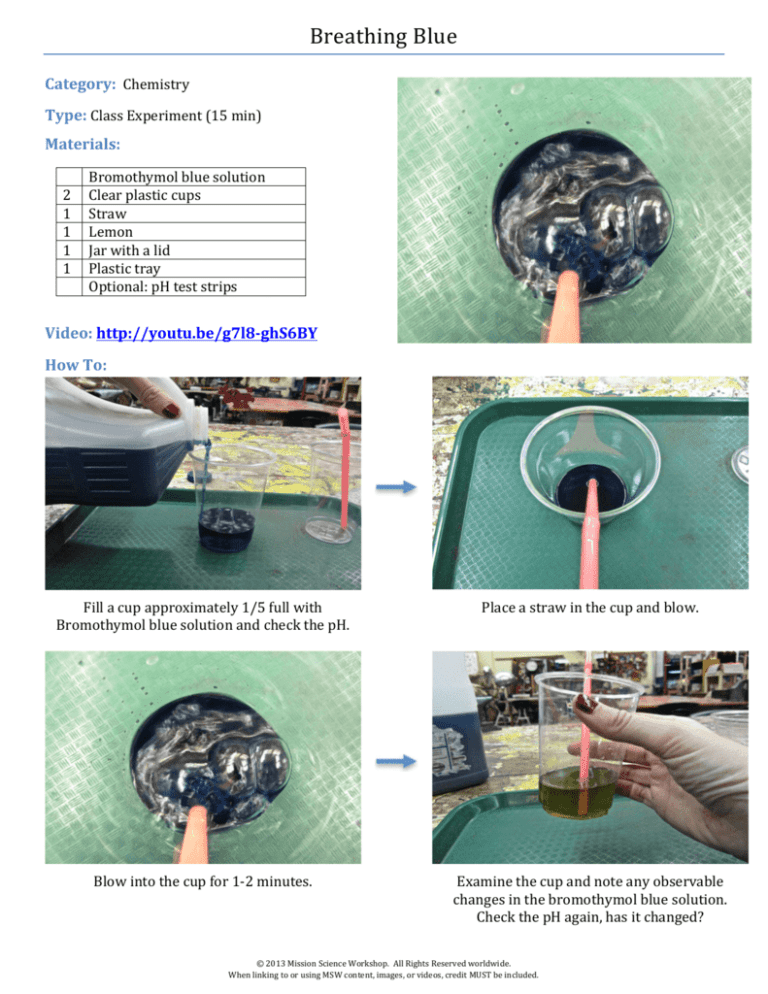
Breathing Blue Category: Chemistry Type: Class Experiment (15 min) Materials: 2 1 1 1 1 Bromothymol blue solution Clear plastic cups Straw Lemon Jar with a lid Plastic tray Optional: pH test strips Video: http://youtu.be/g7l8-­‐ghS6BY How To: Fill a cup approximately 1/5 full with Bromothymol blue solution and check the pH. Place a straw in the cup and blow. Blow into the cup for 1-­‐2 minutes. Examine the cup and note any observable changes in the bromothymol blue solution. Check the pH again, has it changed? © 2013 Mission Science Workshop. All Rights Reserved worldwide. When linking to or using MSW content, images, or videos, credit MUST be included. Pour some bromothymol blue into a new cup. Make a small cut in a lemon. Squeeze 3-­‐4 drops of lemon juice into the cup. Swirl the cup to mix the contents. What happens? Check the pH of the new solution. How is this similar to the previous experiment? Are there any ways to change the color of the solution from yellow back to blue? Place the solution from the breath test into a jar with a lid. Shake for 30 seconds and open the lid. Test the pH and continue to repeat the process. Fine Points: → Bromothymol blue can be found in pet stores. → Bromothymol blue is not considered to be dangerous, however, if it comes into contact with skin the area should be washed immediately. → Let students know that bromothymol blue should not be ingested. → pH test strips are inexpensive and can be found at most drug stores. Testing the pH of solution during the process is not necessary but will help to meet the Next Generation Science Standard 5-­‐PS1 Matter & Its Interactions. Objectives: During this activity students will: 1. Test exhaled breath for carbon dioxide. 2. Learn how to use an indicator as a simple way to determine pH. 3. Determine whether the mixing of two substances results in a new solution. Concepts Involved: •
When carbon dioxide dissolves in water, carbonic acid is formed. © 2013 Mission Science Workshop. All Rights Reserved worldwide. When linking to or using MSW content, images, or videos, credit MUST be included. •
Indicators can be used to determine the pH of solutions. Focus Questions: 1.
2.
3.
4.
5.
6.
7.
Why does the solution change color when we breathe into it? Why does the solution change color when lemon juice is added to it? Did the lemon juice or your breath contain more acid and how could you tell? This experiment produces carbonic acid. Is the change into acid a physical or a chemical change? What are examples of natural and industrial sources of carbon dioxide? How can the solution be changed from yellow/green back to blue? Why might we be concerned about the levels of carbon dioxide in the air? Elaboration: Carbon dioxide is a natural component of Earth’s atmosphere and has an important role in maintaining a livable temperature on the planet. All animals, including humans, produce carbon dioxide when they breathe. This experiment can be used to teach two things: it is an example of a chemical reaction and it shows how carbon dioxide builds up in water. Bromothymol blue is a chemical indicator that is used to identify acids and bases. It is commonly used to measure the pH of fish tanks and pools, measuring from 6.0 (yellow color) to 7.6 (green to blue). In the first experiment we exhaled into the solution. As the carbon dioxide from our breath absorbed into the solution it turned from blue to a yellow/green color, and if we continued blowing it would eventually turn completely yellow. When carbon dioxide reacts with water, carbonic acid is formed. The chemical reaction is: The second experiment is performed with a lemon to show what happens when an acid is mixed with Bromothymol blue. Comparing the results of their exhale and the lemon juice mixing with Bromothymol, students should begin to understand that their exhaled breath forms an acid when mixed with the solution. Most students have heard about global climate change. Climate warming is occurring most drastically around the north and south poles as a result of carbon dioxide, nitrous oxide and methane trapping heat and light from the sun in the atmosphere. Human activities have increased the amount of carbon dioxide in the atmosphere, affecting not only the atmosphere but also the oceans which absorb much of the carbon dioxide. Increased carbon dioxide affects the environments of both plants and animals on land and in the ocean. Links to k-­‐12 CA Content Standards: Grades k-­‐8 Standard Set Investigation and Experimentation: Scientific progress is made by asking meaningful questions and conducting careful investigations. As a basis for understanding this concept and addressing the content in the other strands, students should develop their own questions and perform investigations. Grades k-­‐12 Mathematical Reasoning: © 2013 Mission Science Workshop. All Rights Reserved worldwide. When linking to or using MSW content, images, or videos, credit MUST be included. 1.0 Students make decisions about how to approach problems: 1.1 Analyze problems by identifying relationships, distinguishing relevant from irrelevant information, sequencing and prioritizing information, and observing patterns. 1.2 Determine when and how to break a problem into simpler parts. 2.0 Students use strategies, skills, and concepts in finding solutions: 1.1 Use estimation to verify the reasonableness of calculated results. 1.2 2.2 Apply strategies and results from simpler problems to more complex problems. 1.3 Use a variety of methods, such as words, numbers, symbols, charts, graphs, tables, diagrams, and models, to explain mathematical reasoning. 2.5 Indicate the relative advantages of exact and approximate solutions to problems and give answers to a specified degree of accuracy. 3.0 Students move beyond a particular problem by generalizing to other situations: 3.1 Evaluate the reasonableness of the solution in the context of the original situation. 3.2 Note the method of deriving the solution and demonstrate a conceptual understanding of the derivation by solving similar problems. 3.3 Develop generalizations of the results obtained and apply them in other circumstances. Grade 5 Standard Set 1. Physical Sciences Elements and their combinations account for all the varied types of matter in the world. As a basis for understanding this concept: 1.a. Students know that during chemical reactions the atoms in the reactants rearrange to form products with different properties. Grade 5 Standard Set 2. Life Sciences Plants and animals have structures for respiration, digestion, waste disposal, and transport of materials. As a basis for understanding this concept: 5.b. Students know how blood circulates through the heart chambers, lungs, and body and how carbon dioxide (CO2) and oxygen (O2) are exchanged in the lungs and tissues. Grade 8 Standard Set 5. Reactions Chemical reactions are processes in which atoms are rearranged into different combinations of molecules. As a basis for understanding this concept: 5.a. Students know reactant atoms and molecules interact to form products with different chemical properties. 5.b. Students know the idea of atoms explains the conservation of matter: In chemical reactions the number of atoms stays the same no matter how they are arranged, so their total mass stays the same. Grade 9-­‐12 Standard Set 9. Physiology As a result of the coordinated structures and functions of organ systems, the internal environment of the human body remains relatively stable (homeostatic) despite changes in the outside environment. As a basis for understanding this concept: 9.a. Students know how the complementary activity of major body systems provides cells with oxygen and nutrients and removes toxic waste products such as carbon dioxide. Grades 9-­‐12 Standard Set 8. Structure and Composition of the Atmosphere Life has changed Earth’s atmosphere, and changes in the atmosphere affect conditions for life. As a basis for understanding this concept: © 2013 Mission Science Workshop. All Rights Reserved worldwide. When linking to or using MSW content, images, or videos, credit MUST be included. 8.a. Students know the thermal structure and chemical composition of the atmosphere. 8.b. Students know how the composition of Earth’s atmosphere has evolved over geologic time and know the effect of outgassing, the variations of carbon dioxide concentration, and the origin of atmospheric oxygen. 8.c. Students know the location of the ozone layer in the upper atmosphere, its role in absorbing ultraviolet radiation, and the way in which this layer varies both naturally and in response to human activities. © 2013 Mission Science Workshop. All Rights Reserved worldwide. When linking to or using MSW content, images, or videos, credit MUST be included.










