Downloaded from http://rspb.royalsocietypublishing.org/ on March 6, 2016
An early fossil remora (Echeneoidea)
reveals the evolutionary assembly of the
adhesion disc
rspb.royalsocietypublishing.org
Matt Friedman1, Zerina Johanson2, Richard C. Harrington1, Thomas J. Near3
and Mark R. Graham2
1
Department of Earth Sciences, University of Oxford, South Parks Road, Oxford OX1 3AN, UK
Department of Earth Sciences, The Natural History Museum, Cromwell Road, London, UK
3
Department of Ecology and Evolutionary Biology, Yale University, 165 Prospect Street, New Haven,
CT 06520-8106, USA
2
Research
Cite this article: Friedman M, Johanson Z,
Harrington RC, Near TJ, Graham MR. 2013 An
early fossil remora (Echeneoidea) reveals the
evolutionary assembly of the adhesion disc.
Proc R Soc B 280: 20131200.
http://dx.doi.org/10.1098/rspb.2013.1200
Received: 13 May 2013
Accepted: 20 June 2013
Subject Areas:
evolution, palaeontology, developmental
biology
Keywords:
Acanthomorpha, development, Echeneidae,
Oligocene, ontogeny, Percomorpha
Author for correspondence:
Matt Friedman
e-mail: mattf@earth.ox.ac.uk
Electronic supplementary material is available
at http://dx.doi.org/10.1098/rspb.2013.1200 or
via http://rspb.royalsocietypublishing.org.
The adhesion disc of living remoras (Echeneoidea: Echeneidae) represents
one of the most remarkable structural innovations within fishes. Although
homology between the spinous dorsal fin of generalized acanthomorph
fishes and the remora adhesion disc is widely accepted, the sequence of evolutionary—rather than developmental—transformations leading from one to
the other has remained unclear. Here, we show that the early remora
†Opisthomyzon (Echeneoidea: †Opisthomyzonidae), from the early Oligocene
(Rupelian) of Switzerland, is a stem-group echeneid and provides unique
insights into the evolutionary assembly of the unusual body plan characteristic of all living remoras. The adhesion disc of †Opisthomyzon retains
ancestral features found in the spiny dorsal fins of remora outgroups,
and corroborates developmental interpretations of the homology of individual skeletal components of the disc. †Opisthomyzon indicates that the
adhesion disc originated in a postcranial position, and that other specializations (including the origin of pectination, subdivision of median fin
spines into paired lamellae, increase in segment count and migration to a
supracranial position) took place later in the evolutionary history of remoras.
This phylogenetic sequence of transformation finds some parallels in the
order of ontogenetic changes to the disc documented for living remoras.
1. Introduction
From the orbital asymmetry of flatfishes [1] to the lures of anglerfishes [2], some
of the most striking cranial innovations in vertebrates are found among
acanthomorphs, a diverse clade of teleost fishes containing over 18 000 living
species [3]. One of the most bizarre cranial designs within acanthomorphs is
found in Echeneidae (remoras or sharksuckers), a small clade of commensalists
distinguished by a strongly depressed skull bearing a large, segmented
adhesion disc that is used to fasten to hosts, including whales, turtles, sharks
and other bony fishes [4,5].
The evolutionary roots of the remora suction disc have long been the subject of
scientific debate. Developmental patterns [6–8], revealed in meticulous detail by
recent ontogenetic work [5], combined with the segmented construction of the
adhesion disc [9,10], indicate that this structure is derived from the anterior,
spinous portion of the dorsal fin of other acanthomorphs. Modern outgroups of
remoras bear spiny fins that differ considerably in their architecture, meristic
counts and position from suction discs, leaving many questions about how this
evolutionary transformation occurred. Because most fossil remoras are assigned
to extant genera [11–13], they yield no clues beyond those already provided
by living taxa. Exceptional among these palaeontological examples is the extinct
genus †Opisthomyzon from the early Oligocene (Rupelian; approx. 30 Ma) of
Switzerland. Although it was the first fossil remora to be described as such [14]
and was initially embraced as a potentially pivotal taxon in understanding the
& 2013 The Author(s) Published by the Royal Society. All rights reserved.
Downloaded from http://rspb.royalsocietypublishing.org/ on March 6, 2016
(a) Comparative materials
Our analysis includes all living members of Echeneoidei, which
contains the eight species of Echeneidae, two species of Coryphaenidae (dolphinfishes) and single species of Rachycentridae (cobia)
[18]. We also include two fossil taxa: the Oligocene remora
†Opisthomyzon and the Eocene percomorph †Ductor, which has
been associated with Echeneoidei [19]. More distant relatives of
Echeneoidei are represented by three carangiforms [20]: two members of Carangidae ( jacks) and the single species of Nematistiidae
(roosterfish). All inferred phylogenies were rooted using the
outgroup Pomatomus saltatrix, a non-carangiform and the only
member of Pomatomidae (bluefish), which is argued to branch outside Carangiformes (sensu [20]) on the basis of both morphological
[21] and molecular [3,22] evidence.
Osteological features for fossil and living taxa were assessed
from adult specimens. Owing to the rarity of appropriate material
in systematic collections [5,16], larval and other developmental
characters in modern taxa were scored from detailed, well-illustrated descriptions [5,23]. A complete account of our comparative
sample is given in the electronic supplementary material.
3. Systematic palaeontology
Acanthomorpha Rosen, 1973; Percomorpha Rosen, 1973; Carangiformes Jordan, 1923; Echeneoidei Bleeker, 1852; Echeneoidea
Johnson, 1993; †Opisthomyzonidae Berg, 1940; †Opisthomyzon
Cope, 1889; †Opisthomyzon glaronensis (Wettstein, 1886).
(b) Assembly of phylogenetic dataset
(a) Holotype and referred material
The relationships of remoras and their immediate relatives were
assessed through analysis of anatomical (taken here to include
osteological, soft-tissue and developmental) features coded for
fossil and extant species and molecular genetic characters assessed
for living taxa. The anatomical dataset used here is substantially
revised from that presented by O’Toole [4], with a complete
account of corrections and modifications provided in the electronic
supplementary material. Our matrix contains 108 osteological, two
soft-tissue and nine developmental (larval) features, for a total of
119 anatomical characters. Molecular sequences were obtained
from GenBank for mitochondrial 12S, 16S and ND2 gene regions,
and the nuclear ITS-1 region (GenBank accession nos. FJ374786–
FJ374798, AP004444). These correspond to the sequences used by
Gray et al. [18], plus additional data for Carangoides.
Holotype: Naturhistorisches Museum der Burgergemeinde
Bern, Bern, Switzerland, NMBE 5016633, complete individual
preserved in left-lateral view (counterpart NMBE 5017410).
Referred material: Natural History Museum, London, UK,
NHMUK PV P.1995, impression of right side of skeleton (specimen of †Uropteryx elongatus nomen nudum; see below);
NHMUK PV P.4953, complete individual preserved in rightlateral view; Paläontologisches Institut und Museum, Zurich,
PIMUZ AI 2110, complete individual preserved in left-lateral
view; Sedgwick Museum of Geology, Cambridge, UK,
CAMSM C 31451, disrupted impression of right side of skeleton.
(b) Horizon and locality
(c) Phylogenetic analysis
Phylogenetic analyses were completed using maximum parsimony
and Bayesian inference. Parsimony analyses were conducted in
PAUP* v. 4.0b10 [24] using the branch-and-bound search
Engi Slates, Matt Formation, Canton Glarus, Switzerland.
Early Oligocene (Rupelian), but younger than approximately
32 Ma based on K/Ar and 40Ar/39Ar radiometric dates for
the underlying Taveyannaz formation [31].
2
Proc R Soc B 280: 20131200
2. Material and methods
algorithm, with gaps in molecular sequence data treated as missing information. Support for inferred clades was assessed by
character bootstrapping (10 000 pseudoreplicates).
Bayesian analyses were conducted using a metropolis-coupled
Markov chain Monte Carlo strategy implemented in the program
MRBAYES v. 3.2.1 [25]. A single partition was used for ITS-1, 12S
and 16S rRNA gene regions, and three partitions were used for
the mitochondrial ND2 gene based on codon position. Models
of molecular evolution were determined using the Aikake information criterion (AIC) as implemented in MRMODELTEST v. 2.3
[26] (see the electronic supplementary material, table S1). The optimal partitioning scheme for mitochondrial ND2 was selected from
the log of the harmonic mean of the likelihood values sampled
from the posterior distributions of the two compared MRBAYES
runs [26–28]. Morphological characters were treated with the standard Markov-variable (Mkv) model of Lewis [29], assuming
gamma-shaped rate variation and unordered character state transitions. Posterior trees and model parameters were sampled from
MRBAYES runs of 2.0 107 generations. Burn-in was set at 2.0 106
generations, discarding all trees and parameter values sampled
before the burn-in. Stationarity of the chains and convergence of
the trees and parameter values were determined by plotting the
likelihood score and all other model parameter values against
the generation number using the TRACER v. 1.5 [30]. Convergence
of the runs was also assessed by monitoring the average standard
deviation of the split frequencies between the two independent
runs, assuming that stationarity of chains was achieved when
this value was less than 0.005.
In order to test the sensitivity of our phylogenetic inferences
to sampling disparate types of comparative data, we repeated
our analyses with the following partitions excluded: developmental data (characters 113 –119; coded from literature and
unknown for fossils); molecular data (unknown for fossils);
developmental and molecular data; fossil data (†Opisthomyzon
and †Ductor); fossil and developmental data. Solutions arising
from these pruned datasets are summarized below and in the
electronic supplementary material, tables S1 and S2.
rspb.royalsocietypublishing.org
origin of the group [6,9,10,15–17], †Opisthomyzon has subsequently been overlooked or dismissed as a probable relative
of the living Phtheirichthys, and therefore an anatomically
modern member of the crown clade [4,16].
Using a combined analysis of developmental, anatomical,
fossil and molecular data, we find strong support for the placement of †Opisthomyzon outside the living radiation of remoras,
refuting previous verbal arguments that this fossil genus nests
within the echeneid crown clade [4,16]. More significantly, we
show that the adhesion disc of this genus diverges substantially
from that found in extant remoras, and instead displays
striking positional, meristic and anatomical similarities to the
spiny dorsal fins of outgroup taxa. This fossil evidence provides exceptional corroboration for hypotheses of homology
drawn from painstaking study of recent anatomical and
developmental datasets [5], implies a sequence of character
transformation across phylogeny paralleling that occurring
during ontogeny, and highlights the complementary roles of
palaeontological and developmental data in documenting the
evolutionary assembly of specialized anatomical structures.
Downloaded from http://rspb.royalsocietypublishing.org/ on March 6, 2016
(c) Emended diagnosis
(d) Taxonomic remarks
4. Description and comparison
We focus on the neurocranium and adhesion disc of †Opisthomyzon, and their relevance to documenting the evolutionary
assembly of remora cranial anatomy. Abbreviated descriptions
of the remainder of the skeleton are given for completeness,
and emphasize differences between †Opisthomyzon and
crown-group remoras.
(a) Neurocranium
The skull roof of †Opisthomyzon is broad, like that of living
remoras, but differs from modern examples in a series of critical features. First, the lateral ethmoid does not make a large
contribution to the dorsal margin of the orbit (see the electronic
supplementary material, figure S1), unlike the derived condition characteristic of modern remoras. Second, the dorsal
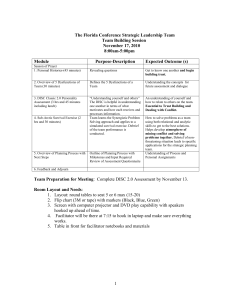
surface of the skull is flat or slightly convex (figure 1a),
unlike the concave surface found in all extant remoras. Third,
the frontals and parietals of †Opisthomyzon bear an irregular
ornamentation (figure 1a,b), similar to that found in Rachycentron but different from the smooth surface that characterizes
modern remoras. Together, these two final features indicate
that the skull roof was covered by only a thin layer of
soft tissue, rather than buried deep within tissue under an
anteriorly positioned adhesion disc.
(b) Adhesion disc
Two specimens of †Opisthomyzon clearly show the adhesion
disc (NMBE 5016633, figure 1a,b; NHMUK PV P.4953,
Proc R Soc B 280: 20131200
Friedman & Johanson [32] mistakenly indicated that
†Opisthomyzon glaronensis is a junior synonym of †Uropteryx
elongatus. The latter is in fact a nomen nudum, because it is
not accompanied by a description, definition or indication,
and therefore fails to satisfy Article 12 of the International
Code on Zoological Nomenclature [33].
We maintain placement of †Opisthomyzon in †Opisthomyzonidae rather than Echeneidae owing to the major morphological
differences between this extinct genus and modern remoras. We
also adopt a modified taxonomic scheme for remoras and their
closest relatives. Johnson [23] identified Echeneidae, Coryphaenidae and Rachycentridae as echeneoids, but did not specify
whether the intended formal name was Echeneoidei (an existing
suborder) or Echeneoidea (a new superfamily). Both have since
appeared in the literature, where their meaning has been identical in terms of composition [4,5,18,34]. We restrict Echeneoidea
to the remora total group, presently comprising Echeneoidei
and †Opisthomyzonidae. Echenoidei contains these families
plus Coryphaenidae and Rachycentridae, matching the most
recent usage of the name [5].
3
rspb.royalsocietypublishing.org
Echeneoidei differing from other members of that group in the
following combination of characters: adhesion disc present and
located over anterior trunk; disc lamellae median (rather than
paired) ossifications in adults; spinous projections absent
from disc lamellae; dermal skull roof strongly ornamented;
anteriorly extensive soft dorsal fin, inserting far in advance of
anterior insertion of anal fin; caudal fin deeply forked.
figure 1c), and their slightly different modes of preservation
provide insight into the anatomy of this feature. We accept
the classical interpretation [10], recently corroborated by
exquisitely detailed ontogenetic and anatomical study [5],
that the lamellae, intercalary bones and interneural rays of
the remora adhesion disc are the homologues, respectively,
of the fin spines, distal radials and proximal-middle radials
of other percomorphs. Of these major components of the
adhesion disc, the lamellae are the most superficial, making
them the easiest to examine in fossil material.
There are several conspicuous differences between the
disc of †Opisthomyzon and that of all crown remoras. First,
it lies posterior to the skull roof in both specimens where it
is preserved (figure 1), confirming positional inferences
based on the anatomy of the skull roof. Second, only six
lamellae appear to be present, corroborating the low counts
provided by previous workers [16]. Third, the lamellae bear
no pectinations along their posterior margin with the exception of the median spinule. Fourth, the lamellae are single,
bilaterally symmetrical structures rather than paired
elements. Fifth, the median spinules are not autogenous
and are instead united with their associated lamella. Sixth,
the disc is short, measuring slightly more than 10 per cent
of standard length (SL), whereas the disc in modern examples
ranges from 18– 28% of SL in Phtheirichthys [35] to over 40%
of SL in some species of Remora [4].
For the first five characters, states observed in †Opisthomyzon correspond to primitive conditions of the adhesion disc
as predicted from dorsal fin structure in outgroups of remoras
and developmental patterns in living echeneids (figures 1b,c
and 2c). The position of the adhesion disc posterior to the
skull in †Opisthomyzon disagrees with the supracranial position
of living remoras, but corresponds with the arrangement found
in generalized percomorphs and, to a lesser degree, that in
early developmental stages in extant remoras, where the disc
initially develops posterior to the orbits only to later extend
to the anterior tip of the snout [5,7,8,36]. Living remoras [35]
and other fossil examples [11,12] generally have discs bearing
anywhere from 15 to 28 lamellae. Phtheirichthys is unusual
among modern remoras in having a count of 9–11 lamellae,
but this small number appears to be secondary based on the
nesting of this genus high within the crown (figure 2a) [18].
By contrast, the low count of lamellae in the adhesion disc of
†Opisthomyzon corresponds closely to the number of dorsal
spines in the closest relatives of remoras that bear distinct spinous and soft portions of the dorsal fin (7–9 in Rachycentron; 7
in †Ductor [19,37]). The absence of posterior pectinations along
individual lamellae in †Opisthomyzon disagrees with the
condition in extant remoras [4,5] and all other fossil representatives of the group with well-preserved adhesion discs [11,12].
However, the arrangement seen in †Opisthomyzon is in accord
with the morphology of dorsal fin spines in outgroups of
remoras, where trailing-edge pectination is absent [5,37]. The
disc lamellae of †Opisthomyzon do not consist of paired right
and left ossifications, as is the case in adult crown-group
remoras [5], but instead consist of a single median bone.
Additionally, the median spinule is united with the lamellae
(figures 1b,c and 2c), rather than appearing as a separate ossification of the sort found in modern adult remoras (figure 2c).
These last two features of the lamellae in †Opisthomyzon draw
immediate comparisons to the unpaired dorsal fin spines of
generalized percomorphs and the condition found in early
developmental stages in extant remoras, where the disc
Downloaded from http://rspb.royalsocietypublishing.org/ on March 6, 2016
4
(a)
rspb.royalsocietypublishing.org
(b)
intb.p
dl.p
dl.m
intb.m
pa (l)
intr
dl.a ?intb.a
10 mm
soc
(c)
pa (r)
intb.m
?intb.a
dl.m
dl.a
?intb.p
pa (l)
epo (l)
ex.2 (l)
10 mm
ex.1 (l)
pto (l)
Figure 1. Anatomy of †Opisthomyzon glaronensis, with an emphasis on structure of the adhesion disc. (a) Specimen NMBE 5016633 in right-lateral view. (b) Region
highlighted by white outline in (a), showing articulated adhesion disc plus the portions of the skull roof. (c) Close-up of specimen NHMUK PV P.4953 in left-lateral
view, showing disrupted adhesion disc and posterior margin of the skull. dl.a, disc lamella from the anterior of the adhesion disc; dl.m, disc lamella from the middle
of the adhesion disc; dl.p, most posterior disc lamella; epo, epiotic; ex.1, first extrascapular; ex.2, second extrascapular; intb.a, intercalary bone from anterior of
adhesion disc; intb.m, intercalary bone from middle of adhesion disc; intb.p, most posterior intercalary bone; intr, interneural rays; pa, parietal; pto, pterotic; soc,
supraoccipital. For paired structures, r and l indicate right or left side, respectively.
lamellae are joined across the midline and are united with the
median spinule [5].
(c) Cheek, jaws, suspensorium and opercular series
The jaws of †Opisthomyzon form a short, beak-like snout. The
premaxilla bears a well-developed ascending process like
that of generalized carangiforms, but which is absent in
crown remoras (see the electronic supplementary material,
figure S1). As in living remoras, the maxilla is slender and
splint-like. The ventral process of the dentary is long, comparable with generalized carangiform conditions, but differing
from the short process characteristic of extant remoras. Oral
teeth in †Opisthomyzon are small, appearing to consist of a
pavement of denticles. This contrasts with the condition in
crown remoras, where long, slender teeth are present [4]. The
circumorbital series of †Opisthomyzon closely resembles that
of living remoras, but obscures details of the suspensorium.
It is clear, however, that the articular surface of the quadrate
is directed anteriorly, matching the derived condition of
extant remoras (see the electronic supplementary material,
figure S1). The opercular series of †Opisthomyzon does not
deviate radically from that found in modern remoras.
(d) Postcranial skeleton
The postcranium shows several proportional differences in
comparison with living remoras (figures 1a and 2b; electronic
supplementary material, figure S2). Principal among these is
the shape of the body, which is relatively deep and fusiform,
unlike the comparatively long and slender postcrania of
extant remoras. This is complemented by the structure of
the median fins. The anterior insertion of the dorsal fin lies
far anterior to that of the anal fin in †Opisthomyzon, whereas
insertion bases are effectively symmetrical in crown remoras.
†Opisthomyzon also bears a caudal fin with a deeply notched
Proc R Soc B 280: 20131200
50 mm
Downloaded from http://rspb.royalsocietypublishing.org/ on March 6, 2016
(a)
(b)
(c)
Pomatomus saltatrix
‘generalized’ carangiforms
Carangoides spp.
Seriola spp.
fs (dl)
†Ductor vestenae
Rachycentron canadum
Echeneoidei
Proc R Soc B 280: 20131200
*
*
intb
Coryphaena hippurus
*
Coryphaena equiselis
†Opisthomyzon glaronensis
Phtheirichthys lineatus
Echeneoidea
* Echeneis naucrates
intb
Remora albescens
*
Remora australis
0.07
*
posterior
probability
=1
= 0.99–0.9
= 0.89–0.70
dl
* Echeneis neucratoides
*
Remora remora
Remora osteichir
dl
Remora brachyptera
crown-group remoras
Figure 2. Phylogenetic placement of †Opisthomyzon and the stepwise origin of the remora adhesion disc. (a) Phylogeny for remoras and their closest relatives based
on Bayesian inference analysis of ‘total-evidence’ dataset (morphology þ development þ fossils þ genetic sequences). Branch lengths are scaled to the number of
changes occurring along them. Discs on nodes indicate the frequency of clades in posterior samples, and clades receiving bootstrap support in excess of 95% in a
maximum-parsimony total-evidence analysis are indicated with an asterisk. Other trees and character optimizations provided in the electronic supplementary
material. (b) Carangiform body plans (from top to bottom): a generalized carangiform (the carangid Elagatis), the stem-group remora †Opisthomyzon and a
crown-group remora (Remora). (c) Anatomy of the spine (¼ disc lamella of remoras) and distal radial (¼ intercalary bone of remoras) corresponding to the profiles
shown in (b). dl, disc lamella; dr (intb), distal radial (homologue of intercalary bone); fs (dl), fin spine (homologue of disc lamella); intb, intercalary bone. F in
components of ‘generalized’ carangiform based on Berry [44]; those of crown-group remora based on Britz & Johnson [5].
posterior margin (figure 1a), which differs from the gently
concave to convex profile that characterizes the caudal fins
of modern remoras. The vertebral column of †Opisthomyzon
comprises 10 abdominal and 13 caudal centra. Each centrum
is anteroposteriorly elongate. †Opisthomyzon lacks the
derived, laterally directed parapophyses common to living
remoras [4] and all other fossil examples [11,12]. The body
of †Opisthomyzon is covered with small, diamond-shaped
cycloid scales (figure 1a; electronic supplementary material,
figures S1 –S3).
In all specimens, the pectoral girdle is largely concealed
by opercular bones. However, details of the pelvic girdle
are apparent. The girdle in †Opisthomyzon is narrow in comparison with its length, unlike the broad pelvic girdle
common to extant remoras. However, the presence of a
medial anterior arm of the girdle represents a derived feature
linking †Opisthomyzon to modern remoras (see the electronic
supplementary material, figure S3).
5. Evolutionary relationships
All phylogenetic analyses agree in the placement of †Opisthomyzon on the remora stem as the immediate sister group of
rspb.royalsocietypublishing.org
Nematistius pectoralis
5
dr (intb)
crown echeneids ( posterior probability ¼ 1 in Bayesian analyses of complete datasets and all pruned datasets that
include fossils; clade recovered in 100% of bootstrap replicates in parsimony analyses of both complete dataset and
all pruned datasets that include fossils; figure 2a; electronic
supplementary material, figures S4–S6 and tables S1 and
S2). This genus shares with living remoras a series of unambiguous morphological synapomorphies, the most obvious of
which is the adhesion disc. However, †Opisthomyzon retains
several primitive characters not found in living remoras.
Most striking are the fusion of disc lamellae along the midline
(i.e. the presence of true dorsal fin spines), low number of
disc lamellae comparable with the number of fin spines in
living outgroups of remoras and absence of pectination,
which are joined by a series of generalized features found
elsewhere in the skeleton, including: placement of the spinous component of the dorsal fin posterior to the skull;
lateral ethmoid not contributing to dorsal margin of orbit;
dorsally convex, ornamented frontals bearing a sensory-line
canal on the body of the bone rather than the margin; a
small median ethmoid; an ascending process of the premaxilla; dorsal and ventral processes of the dentary of equal
length; weakly developed anterior wing of the preopercle;
presence of two postcleithra; a long pelvic girdle; and lack
Downloaded from http://rspb.royalsocietypublishing.org/ on March 6, 2016
The remora adhesion disc is one of the most striking anatomical specializations of living vertebrates, but the consistent
anatomy of this structure among modern species does not
provide a clear picture of its evolutionary history. Detailed
anatomical and developmental study has delivered compelling evidence for the homology between components of
generalized percomorph dorsal fins and remora discs [5],
but does not provide direct evidence for the sequence of evolutionary events by which one was transformed into the other.
This problem is not unique to the remora adhesion disc.
Many groups of teleosts are characterized by elaborate anatomical innovations absent in their closest living relatives.
Fossils play a particularly important role in such cases and
can deliver unique insights into the evolutionary origin of
extreme morphological specializations [1,38 –40].
The conclusion that †Opisthomyzon is a stem-group remora,
combined with novel anatomical information arising from the
discovery and preparation of additional specimens, provides
the first clues about the sequence of evolutionary changes
that led to the assembly of the adhesion disc. Mapping
Acknowledgements. We thank R. Arrindell, B. Brown (American Museum
of Natural History), H. López-Fernández (Royal Ontario Museum),
P. Campbell (NHMUK) and S. Raredon (National Museum of Natural
History, Smithsonian) for providing access to Recent comparative
material, H. Furrer (PIMZ), U. Menkveld (NMBE) and M. Riley
(CAMSM) for access to fossil specimens, J. McDowell (Virgina Institute
of Marine Science) for providing previously published molecular
sequence alignments, and R. Britz (NHMUK) for conversations
about the structure, development and homology of the remora
adhesion disc. H. Taylor (NHMUK) took photographs of fossil specimens. M. V. H. Wilson (University of Alberta) and an anonymous
reviewer provided insightful comments that improved this contribution, while C. Kammerer (Museum für Naturkunde) clarified the
taxonomic status of the remora from the Engi Slates.
Funding statement. Financial support was provided by the John Fell
Fund, St Hugh’s College and NERC grant no. NE/J022632/1 to
M.F. and Z.J.
6
Proc R Soc B 280: 20131200
6. Discussion
character states on our robustly supported phylogenies provides two-stage resolution relating to the evolution of this
remarkable structure. It is clear that many key transformations,
most notably modification of fin spines into laterally expanded
lamellae, took place while the disc occupied a postcranial position. At this stage, lamellae were still joined along the midline,
comparable with the condition of dorsal fin spines in generalized percomorphs. The second stage was characterized by
anterior migration of the disc, the separation of lamellae into
paired ossifications, the development of pectination along
the posterior margins of the lamellae and an increase in the
number of segments in the disc. Even at this coarse level of resolution, is it apparent that there are clear parallels between the
ontogenetic origin of the adhesion disc and its sequence of
phylogenetic transformation, the clearest of which are the
migration of the disc on to the cranium, delayed division of
individual lamellae and the late origin of spinules in both
sequences [5]. However, disagreements in the timing of
specific events between these two trajectories, such as the
expansion of the most posterior laminae and intercalary
bones only after migration of the disc on to the skull during
the development of living forms [5], indicate that one cannot
be read as a surrogate for the other.
Many striking anatomical innovations of acanthomorphs—
from the asymmetry of flatfishes to the bills of swordfishes to
the lures of anglerfishes—appear to have arisen as part of a
large-scale morphological radiation in the latest Late Cretaceous
and earliest Palaeogene [3,41]. By contrast, the remora adhesion
disc would seem to be a comparatively modern innovation. The
early Oligocene †Opisthomyzon, along with roughly coeval
material provisionally assigned to the extant genus Echeneis
[11,12], represent the earliest known remoras. Rachycentrids
and coryphaenids have no fossil record, and no remoras
of Eocene or earlier age are known. The earliest representative
of Echeneoidei is the early Eocene †Ductor, and the oldest
carangiforms are jacks from the late Palaeocene [42]. This
palaeontological timescale agrees with that of recent molecular
clock analyses that place the origin of the remora total group—
and therefore the earliest possible date for the origin of the
adhesion disc—in the mid-late Eocene [3]. This interval has
historically been poorly sampled for articulated marine
acanthomorphs [41], but excavation of the few known deposits
of this age continue to yield new taxa [43], raising the possibility
that future work might uncover deeper branches of the remora
stem group that will add further detail to our understanding of
the evolution of the remarkable adhesion disc.
rspb.royalsocietypublishing.org
of greatly expanded horizontal parapophyses (see the electronic
supplementary material, figures S5 and S6). The monophyly of
crown-group remoras to the exclusion of †Opisthomyzon is
strongly supported (posterior probability ¼ 1 in all Bayesian
analyses including fossils; clade recovered in 100% of bootstrap replicates in all parsimony analyses including fossils;
figure 2a; electronic supplementary material, figure S4 and
tables S1 and S2).
Significantly, most of our analyses provide strong support
for the hypothesis that remoras form the sister group to a
clade comprising Coryphaena and Rachycentron, consistent
with published molecular phylogenies [3] and arguments
made on the basis of larval development [23]. This contrasts
with recent cladistic treatments of anatomical data [4] and classical schemes [10], both of which have aligned Rachycentron
with remoras to the exclusion of Coryphaena. The historical
association of Rachycentron and remoras reflects conspicuous
phenotypic similarities shared between these groups, but we
argue that many of these traits are more general in their distribution. Most remoras and Rachycentron share a broadly
similar external appearance, with a relatively slender body
and darkly pigmented brown-to-black dorsal surface [36,37]
that strongly contrasts with the vibrant coloration and distinctive body outline characteristic of Coryphaena. The early
Eocene †Ductor, from the Lagerstätte at Bolca, Italy, shares
with remoras and Rachycentron a slender postcranium with
a darkly pigmented dorsal surface (e.g. NHMUK PV
P.1987; electronic supplementary material, figure S7). Placement of †Ductor on the stem of Echeneoidei or as sister
to Rachycentridae þ Coryphaenidae in all of our analyses
suggests that these characteristics are simply symplesiomorphies of crown Echeneoidei, and therefore have no bearing
on the relationships between remoras and Rachycentron. We
only recover a sister-group relationship between remoras and
Rachycentron when both larval and molecular characters are
ignored in maximum-parsimony analyses, or when molecular
data, or these in combination with larval characters, are excluded
from Bayesian analyses (see the electronic supplementary
material, tables S1 and S2).
Downloaded from http://rspb.royalsocietypublishing.org/ on March 6, 2016
References
2.
3.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15. Cope ED. 1889 Storms on the adhesive disk of
Echeneis. Am. Nat. 23, 254–255.
16. Gudger EW. 1926 A study of the smallest shark-suckers
(Echeneididæ) on record, with special reference to
metamorphosis. Am. Mus. Novit. 234, 1–26.
17. Norman JR. 1939 Remoras or sucking-fishes. Nature
143, 52 –55. (doi:10.1038/143052a0)
18. Gray KN, McDowell JR, Collette BB, Graves JE. 2009
A molecular phylogeny of the remoras and their
relatives. Bull. Mar. Sci. 84, 183–198.
19. Blot J. 1969 Les poissons fossiles du Monte Bolca.
Classés jusqu’ici dans les familles des Carangidae
Menidae Ephippidae Scatophagidae. Stud. Ric.
Giaciam. Terz. Bolca 1, 1 –525.
20. Wiley EO, Johnson GD. 2010 A teleost classification
based on monophyletic groups. In Origin and
phylogenetic interrelationships of teleosts (eds JS
Nelson, H-P Schultze, MVH Wilson), pp. 123–182.
Munich, Germany: Verlag Dr. Friedrich Pfeil.
21. Johnson GD. 1986 Scombroid phylogeny: an
alternative hypothesis. Bull. Mar. Sci. 39, 1–41.
22. Orrell TM, Collette BB, Johnson GD. 2006 Molecular
data support separate scombroid and xiphioid
clades. Bull. Mar. Sci. 79, 505 –519.
23. Johnson GD. 1984 Percoidei: development and
relationships. In Ontogeny and systematics of fishes
(eds HG Moser, WJ Richards, DM Cohen, MP Fahay,
AW Kendall, SL Richardson), pp. 464 –498.
Lawrence, KS: Allen Press.
24. Swofford DL. 2002 PAUP*. Phylogenetic analysis
using parsimony (*and other methods), v. 4.
Sunderland, MA: Sinauer Associates.
25. Ronquist F et al. 2012 MrBayes 3.2: efficient
Bayesian phylogenetic inference and model choice
across a large model space. Syst. Biol. 61, 539– 542.
(doi:10.1093/sysbio/sys029)
26. Nylander JAA. 2004 MrModeltest v2. Program
distributed by the author. Uppsala, Sweden:
Evolutionary Biology Centre, Uppsala University.
27. Newton MA, Raftery AE. 1994 Approximate Bayesian
inference with the weighted likelihood bootstrap.
J. R. Stat. Soc. Ser. B. Methodol. 56, 3– 48.
28. Brandley MC, Schmitz A, Reeder TW. 2005
Partitioned Bayesian analyses, partition choice, and
the phylogenetic relationships of scincid lizards.
Syst. Biol. 54, 373–390. (doi:10.1080/
10635150590946808)
29. Lewis PO. 2001 A likelihood approach to estimating
phylogeny from discrete morphological character
data. Syst. Biol. 50, 913–925. (doi:10.1080/
106351501753462876)
30. Rambaut A, Drummond AJ. 2003 Tracer, MCMC trace
analysis package. See http://beast.bio.ed.ac.uk/Tracer.
31. Gasser D, den Brok B. 2008 Tectonic evolution of the
Engi Slates, Glarus Alps, Switzerland. Swiss
J. Geosci. 101, 311–312. (doi:10.1007/s00015-0081258-0)
32. Friedman M, Johanson ZA. 2012 †Opisthomyzon
glaronensis (Wettstein, 1886) (Acanthomorpha:
†Opisthomyzonidae), a junior synonym of
†Uropteryx elongatus Agassiz, 1844. J. Vertebr.
Paleontol. 32, 1202 –1206. (doi:10.1080/02724634.
2012.684817)
33. International Commission on Zoological
Nomenclature. 1999 International code of zoological
nomenclature. London, UK: The International Trust
for Zoological Nomenclature, The Natural History
Museum.
34. Johnson GD. 1993 Percomorph phylogeny: progress
and problems. Bull. Mar. Sci. 52, 3 –28.
35. Lachner EA. 1986 Echeneididae. In Fishes of the
north-eastern Atlantic and the Mediterranean (eds
PJP Whitehead, ML Bauchot, J-C Hureau, J Nielsen,
E Tortonese), pp. 1329–1334. Paris, France:
UNESCO.
36. Nakajima H, Kawahara H, Takamatsu S. 1987 The
breeding behavior of larvae and juveniles of the
sharksucker, Echeneis naucrates. Jpn. J. Ichthyol. 34,
66– 70. (doi:10.1007/BF02904145)
37. Collette BB. 2003 Rachycentridae. In FAO species
identification guide for fishery purposes: the living
marine resources of the western Central Atlantic.
Bony fishes part 2 (Opistognathidae to Molidae), sea
turtles and marine mammals, vol. 3 (ed. KE
Carpenter), pp. 1420–1421. Rome, Italy: Food and
Agriculture Organization of the United Nations.
38. Tyler JC, Bannikov AF. 1992 New genus of primitive
ocean sunfish with separate premaxillae from the
Eocene of southwest Russia (Molidae,
Tetraodontiformes). Copeia 1992, 1014 –1023.
(doi:10.2307/1446631)
39. Arratia G. 1997 Basal teleosts and teleostean
phylogeny. Palaeo Ichthyol. 7, 1–168.
40. Belouze A. 2002 Compréhension morphologique et
phylogénétique des taxon actuels et fossils rapports
aux Anguilliformes (‘poissons’, téléostéens). Docum.
Lab. Géol. Lyon 158, 1–197.
41. Friedman M. 2010 Explosive morphological
diversification of spiny-finned teleost fishes in the
aftermath of the end-Cretaceous extinction.
Proc. R. Soc. B 277, 1675– 1683. (doi:10.1098/rspb.
2009.2177)
42. Bannikov AF, Parin NN. 1997 The list of marine
fishes from Ceonzoic (upper Paleocene-middle
Miocene) localities in southern European Russia and
adjacent countries. J. Ichthyol. 37, 150 –155.
43. Bannkov AF, Carnevale G, Parin NV. 2011 The
new family Caucasichthyidae (Pisces, Perciformes)
from the Eocene of the North Caucasus.
Paleontol. J. 45, 83– 89. (doi:10.1134/
S0031030111010047)
44. Berry FH. 1969 Elagatis bipinnulata (Pisces:
Carangidae): morphology of the fins and other
characters. Copeia 1969, 454 –463.
Proc R Soc B 280: 20131200
4.
Friedman M. 2008 The evolutionary origin of flatfish
asymmetry. Nature 454, 209– 212. (doi:10.1038/
nature07108)
Pietsch TW, Orr JW. 2007 Phylogenetic relationships
of deep-sea anglerfishes of the suborder Ceratioidei
(Teleostei: Lophiiformes) based on morphology.
Copeia 2007, 1–34. (doi:10.1643/00458511(2007)7[1:PRODAO]2.0.CO;2)
Near TJ et al. In press. Phylogeny and tempo of
diversification in the superradiation of spiny-rayed
fishes. Proc. Natl Acad. Sci. USA.
O’Toole B. 2002 Phylogeny of the species of the
superfamily Echeneoidea (Perciformes: Carangoidei:
Echeneidae, Rachycentridae, and Coryphaenidae),
with an interpretation of echeneid hitchhiking and
behaviour. Can. J. Zool. 80, 596–623. (doi:10.
1139/z02-031)
Britz R, Johnson GD. 2012 Ontogeny and homology
of the skeletal elements that form the sucking disc
of remoras (Teleostei, Echeneoidei, Echeneidae).
J. Morph. 273, 1353 –1366. (doi:10.1002/
jmor.20063)
Tåning AV. 1926 Position du disque céphalique chez
les Échénéides au cours de l’ontogénèse. C. R. Hebd.
Acad. Sci. 182, 1293 –1295. (doi:10.1080/
00222938809460878)
Akazaki M, Nakajima H, Kawahara H, Takamatsu S.
1976 Embryonic development and metamorphosis
after hatching in the sharksucker, Echeneis
naucrates. Jpn. J. Ichthyol. 23, 153–159. (doi:10.
1007/BF02904145)
Richards WJ. 2006 Echeneidae: remoras. In Early
stages of Atlantic fishes: an identification guide
for the North Atlantic, vol. II (ed. WJ Richards),
pp. 1433 –1438. Boca Raton, FL: CRC Press.
Storms R. 1888 The adhesive disc of Echeneis. Ann.
Mag. Nat. Hist. 2, 67 –76. (doi:10.1080/
00222938809460878)
Regan CT. 1912 The anatomy and classification of
the teleostean fishes of the order Discocephali. Ann.
Mag. Nat. Hist. 10, 634–637.
Danil’chenko PG. 1960 Kostistye ryby Maikopskikh
otlozhenii Kavkaza [Bony fishes of the Maikop
deposits of the Caucasus]. Trudy Paleontol. Inst.
Akad. Nauk SSSR 78, 1– 208.
Micklich N. 1998 New information on the
fishfauna of the Frauenweiler fossil site.
Ital. J. Zool. 65, 169–184. (doi:10.1080/
11250009809386809)
Kotlarczyk J, Jerzmańska A, Świdnicka E,
Wiszniowska T. 2006 A framework of ichthyofaunal
ecostratigraphy of the Oligocene-early Miocene
strata of the Polish outer Carpathian basin. Ann. Soc.
Geol. Pol. 76, 1 –111.
Wettstein A. 1886 Ueber die Fischfauna des
tertiaeren Glarnerschiefers. Abh. Schweiz. Paläontol.
Gesell. 13, 1–103.
rspb.royalsocietypublishing.org
1.
7