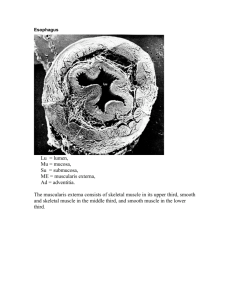
Peroxisomes: family of versatile organelles
advertisement

Kontinkangas, L101A Biochemistry of cellular organelles Lectures: 1. Membrane channels; 2. Membrane transporters; 3. Soluble lipid/metabolite-transfer proteins; 4. Mitochondria as cellular organelles; Seminar: Isolation of subcellular organelles; 5. Mitochondrial inheritance; 6. Mitochondria in health and disease; 7. Endoplasmic Reticulum (ER) and lipids; 8. Structure and function of peroxisomes; Seminar: Mitochondria in cellular life. Dr. Vasily Antonenkov, Visiting professor Dept. Biochemistry, Oulu University Oulu, Finland Web site: 1 Lecture 8: Structure and function of peroxisomes 1. 2. 3. 4. 5. 6. 7. 8. History and terminology; Morphology; Function of peroxisomes in mammals; Induction of peroxisomes by fibrates and lipids; Diversity of peroxisomal functions; Biogenesis of peroxisomes; Peroxisomal disorders; Channels and transporters in peroxisomes. History and terminology • • • • First enzymes discovered in the particles: catalase and H202-producing oxidases (urate oxidase, D-amino acid oxidase and glycolate oxidases); Catalase is able to catalyze two reactions: dismutation of H202: 2H202=H20 + 02 and peroxidation (if substrate is available) of some compounds (methanol, ethanol, certain phenols, formaldehyde, formic acid, and the nitrite ion): The oxidases produce H202 that can be used by catalase to oxidize corresponding substrates. The proposed chain of reactions gives the name to the organelles: peroxisomes, i.e. particles were hydrogen peroxide is metabolized. Terminology • Peroxisomes and their relatives form family of organelles called microbodies. In addition to peroxisomes this family contains: • Plant seeds: Glyoxisomes. These particles contain in addition to catalase and oxidases the enzymes involved in glyoxylate cycle; Trypanosome species (parasites in blood causing some diseases like sleeping disease): Glycosomes. The particles contain glycolytic pathway enzymes side by side with proteins characteristic for other microbodies; • • Microperoxisomes: small peroxisomes detected mainly morphologically in all mammalian tissues except liver and kidney where large peroxisomes are present; • Woronin bodies: special type of microbodies in fungi (mushrooms) dealing with repearing of wounded roots. Morphology • • Mammalian liver or kidney peroxisomes are globular structures approximately 0.5 micrometers in diameter, surrounded by a single membrane. Liver and kidney peroxisomes frequently contain electron dense structure called nucleoid – natural crystal of the enzyme urate oxidase. • Rat liver peroxisomes (two different stainings): Microperoxisomes • Most peroxisomes in plants and yeasts are with size around 0.5 mkm. In contrast, peroxisomes in mammalian tissues (except for liver and kidney) are small (0.1-0.2 mkm) and without nucleoid. It is difficult to recognize them on tissue slices using common transmission electron microscopy. • Method has been developed (Graham, Karnovsky staining) to detect catalase in peroxisomes by its peroxidase activity with diaminobenzidine. The product of this reaction forms an insoluble sediment which can be seen under EM. • Rat parotid exocrine glands (photo). Function of peroxisomes (I) • The main function of peroxisomes is an oxidative degradation of compounds with poor (low) solubility in water and in lipids as well. Most of these compounds are amphipathic molecules (mostly lipids) containing a charged group and a large hydrophobic part, such as long- (C16-C20) and very long- (C22 and longer) fatty acids, bile acids, some amino acids, etc. Some non-lipid molecules with poor solubility in water: purines and oxalates, are also oxidized in peroxisomes . Function of mammalian peroxisomes (II) • One example how mammalian peroxisomes can participate in a complex degradation pathway: formation of bile acids from cholesterol. Function of mammalian peroxisomes (III) • The main function of mammalian peroxisomes is the beta-oxidation of long- and very long-chain fatty acids and the side chain of bileacid precursors. Dual localization of the beta-oxidation in mammals (peroxisomes and mitochondria). • The peroxisomal beta-oxidation of fatty acids does not proceed to completion, i.e., complete degradation of fatty acids to acetyl (C2) groups. Instead, it produces chain-shortened fatty acids (C6-C14) which can be used inside or outside peroxisomes for synthesis of some compounds or exported to mitochondria for further oxidation. Function of mammalian peroxisomes (IV) • • Alpha-oxidation of branchedchain fatty acids derived from phytol – constituent of plant chloroplast; Oxidation of glycolic acid, main source of it is plant leafs and other green staff. Function of mammalian peroxisomes (V) • Catabolism of purines - another example how peroxisomes participate in a complex metabolic pathway. Proliferation of mammalian peroxisomes (I) • Some amphipathic compounds increase amount and size of peroxisomes in liver, kidney and intestine of rodents (mice, rats), and, in a less extend, in humans. Proliferation of peroxisomes leads to activation of peroxisomal beta-oxidation of long chain fatty acids in the liver for more than 10 times (rodents). • The natural peroxisome proliferators are most probably some long chain fatty acids, especially unsaturated acids. • The proliferation of peroxisomes is an adaptation mechanism to excessive consumption of lipids. It is a part of more complex system for regulation of lipid metabolism. Proliferation of mammalian peroxisomes (II) Mitochondrial beta oxidation is also increased, but at the lower level. Proliferation of mammalian peroxisomes (III) • Receptor conception: cytosolic receptor binds ligand (peroxisome proliferator) and deliver it to the nucleus where the complex interacts with DNA and activates transcription of peroxisomal genes. Peroxisome proliferator-activated receptor family of proteins - PPAR’s. Only PPAR alpha is responsible for proliferation of peroxisomes, other members of the family are involved in different steps of regulation of the lipid metabolism and in cells growth and differentiation. Proliferation of mammalian peroxisomes (IV) • After ligand binding the PPAR’s heterodimerize with another nuclear receptor, the retinoid-X receptor (RXR), that binds retinoic acid (related to vitamin A), and next, the PPAR/RXR complex binds to DNA sequences containing direct repeats of the hexanucleotide AGGTCA, known as direct repeat 1 (DR-1) response element. These repeats are present in the promoter regions of PPAR target genes. Some additional proteins (co-activators) are involved in the binding. Diversity of peroxisomal functions (I) • Peroxisomes in plant leafs participate in photorespiration producing link between chloroplasts and mitochondria. • Photorespiration is a lightdependent uptake of O2 and release of CO2. Photorespiration regulates efficiency of photosynthesis that is important at high level of illumination. • Role of peroxisomes: prevent formation of H2O2 and glyoxylate in mitochondria and in chloroplasts Glyoxysomes in plant seeds • Plants can convert lipids to sugars using glyoxylate cycle that is short variant of the citric acid cycle. Animal cells can not carry out the net synthesis of carbohydrate from fat because they have no the glyoxylate cycle. Woronin bodies Like plants, fungi (mushrooms), contain a network of roots that are named hyphae. These roots are separated into sections that connected to each other by pore. In the case of membrane damage in the one section, the pore is sealed by Woronin bodies (like stopper seals bottle) that leads to separation of hyphae sections preventing gross leakage of cytoplasm. Woronin bodies are close relatives to peroxisomes. Both particles have the same biogenesis mechanism, similar morphology and share homologous proteins. However, Woronin bodies contain the only few proteins. Biogenesis of peroxisomes • Biogenesis of peroxisomes is a complex process in which participate of more than 30 different proteins called Pex proteins. • Peroxisomes have no its own DNA, therefore matrix and membrane proteins are synthesized on free (poly)ribosomes in cytoplasm and imported into peroxisomes by special mechanisms. • Several steps in biogenesis: 1. Budding of a new peroxisome from special subdomain of endoplasmic reticulum; 2. Growth and maturation of the peroxisome which involves delivery of the newly synthesized proteins into the particles; 3. Division of the matured peroxisome into daughter peroxisomes Protein import into peroxisomal matrix • Transport of folded, co-factor-bound and oligomeric proteins by shuttling receptors pex5 (PTS1) and pex7(PTS2). • Receptors recognize newly synthesized proteins in the cytosol using peroxisomal targeting signals (PTS): PTS1 is at the C-terminus with a consensus sequence (S/A/C)(K/R/H)(L/I); PTS2 is near the N-terminus with a consensus sequence (R/K)(L/V/I)-xxxxx(H/Q)(L/A) (only few proteins). • After delivering of proteins into peroxisomes, the receptors move back to cytosol. Transfer of proteins across the peroxisomal membrane • • The problem is to deliver folded and even oligomeric protein across the membrane into the matrix. Hypothesis: Transient pore model. When Pex 5 receptor protein together with cargo protein reach the peroxisomal membrane, the Pex 5 forms temporal channel which allows matrix proteins penetrate the membrane. Peroxisomal disorders • Group of inherited diseases in humans caused by malfunctioning of peroxisomes. • Two groups: peroxisome biogenesis disorders caused by malfunctioning of the Pex proteins (example: Zellweger syndrome, cerebro-hepato-renal syndrome caused by deficiency of the pex5 receptor) and the single peroxisomal enzyme deficiencies (example: Refsum disease caused by mutation in the gene coding for phytanoyl-CoA hydroxilase, the key enzyme in alpha oxidation of branched chain fatty acids). • Zellweger syndrome is lethal at early age. Most peroxisomal matrix enzymes are not imported into the particles and degraded in the cytosol after the synthesis or ineffective in their function. The biochemical aberrations observed in Zellweger patients includes accumulation of longand very long fatty acids, branched fatty acids, precursors of bile acids and decrease in formation of bile acids and plasmalogenes. Mechanism of solute transfer across peroxisomal membrane is quite unusual a • • • Contrary to other biological membranes which are open or closed for all solutes, the peroxisomal membrane is freely permeable to small solutes but has very low permeability to molecules comparable by size with cofactors and ATP (‘bulky’ solutes); The peroxisomal membrane may contain non-selective channels (porins) side by side with transporters specific for ‘bulky’ solutes like ATP and NAD transporters. NADH ATP Uric acid Ps Glycine Cyt Transporter Peroxisomes contain their own pool of cofactors but share common pool of small solutes with surrounding cytoplasm. Channel • Transport of lipids (fatty acids and bile acids precursors) into peroxisomes is catalyzed by ABC transporters: three proteins are known, defect of one of them causes an inherited disease – Xlinked adrenoleukodystrophy when very long-chain fatty acids are accumulated in the organism. • Transporters form homodimers and accept CoA derivatives of different lipids as substrates. However, they transfer free lipids (without CoA). The lipids are activated back to CoA derivatives inside peroxisomes by action of acyl-CoA synthase. • How lipid products of peroxisomal metabolism (bile acids, long-chain fatty acids and others) are exported from the particles is unclear. It might be that some ABC transporters work as exporters or some still undiscovered transporters present in the membrane. Peroxisomal lipid transporters Peroxisomal ATP/AMP antiporter • Homologous to mitochondrial ATP/ADP antiporter. • Mice with deleted ATP transporter show no phenotype at normal conditions, but sick after eating phytol – branched-chain alcohol. • Antiporter participates in oxidation of branched chain fatty acids by providing ATP for synthesis of acyl-CoA derivatives by acyl-CoA synthase located inside peroxisomes. • Transporters specific for cofactors (NAD, FAD, CoA) have been recently discovered in mammalian and plant peroxisomes. They are homologous to mitochondrial carriers. Properties of peroxisomal membrane channels • • • • • • All peroxisomal membrane channels have been described in Oulu; The channel proteins belong to two families: Pxmp2p and Pex11p, respectively; In mammals up to four different channel types may be present in the same peroxisomal membrane. Why so many? – is not clear; It seems that all channels are nonselective with a pore diameter up to 1.5 nm; The channels function as a sizeselective filters with an exclusion limit of about 400-500 Da for hydrophilic solutes; Pex11 proteins in addition to their channel-forming activity, are involved in biogenesis of peroxisomes Predictions based on the properties of peroxisomal channels B • • • The channels allow function of the shuttle mechanism for oxidation of NADH and reduction of NADP+ inside peroxisomes. Due to presence of the channels the peroxisomal membrane do not require transporters specific for shuttle molecules like in the case of mitochondria; Presence of channels may explain function of nudix hydrolases localized inside peroxisomes. These enzymes cleave cofactors (NAD/P, CoA) exactly in the middle of the molecule producing smaller compounds which are able to penetrate the membrane through peroxisomal channels. Cofactors are delivered into peroxisomes by specific transporters. Ps Cyt + NAD -oxidation Pyruvate, Dihydroxyacetone phosphate NADH LDH, G-3-PDH NAD Lactate, Glycerol-3phosphate + C Ps Cyt + NAD(P) Nudix hydrolases CoA Overview of the transport machinery in peroxisomal membrane • ABC transporters – import of lipids in peroxisomes; • ATP/AMP antiporter – import of ATP into peroxisomes, export of AMP (?); Cofactor transporters - import of cofactors into peroxisomes; • • Non-selective channels – transfer of small solutes in- and out of peroxisomes; • Channels + nudix hydrolases – removing of cofactors out of peroxisomes; • • Still not known: - export of lipids out of peroxisomes (carnitine transporter?); Suggested questions • • • • • • • • • • • • • • • • What organelles comprise family of microbodies? Describe shortly morphology of mammalian peroxisomes and microperoxisomes; Degradation of what kind of molecules takes place mainly in peroxisomes? Some examples; Describe shortly the main metabolic functions of mammalian peroxisomes; Beta-oxidation of fatty acids in mammalian peroxisomes; What is the difference between alpha- and beta-oxidation of fatty acids? Mechanism of proliferation of mammalian peroxisomes; What receptors are involved in the proliferation of peroxisomes? How they act? What is the main function of peroxisomes in plant leaves and in glyoxysomes in plant seeds? Woronin bodies – what is it? Describe shortly biogenesis of peroxisomes; What is the role of Pex5 and Pex7 proteins in biogenesis of peroxisomes; Two types of peroxisomal disorders – describe shortly; What is the difference in transfer of small solutes and cofactors across peroxisomal membrane? Lipid transporters in peroxisomes; Properties and functional role of peroxisomal membrane channels.