Borohydride Reduction of Ketones: Stereochemistry
advertisement

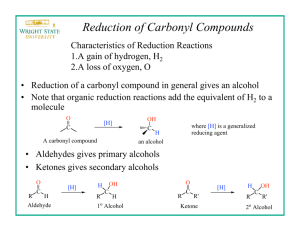
Student Handout Borohydride Reduction of Ketones: Stereochemistry of Estrone Reduction Required Reading: Chapter 19 in Organic Chemistry by G. Marc Loudon (4th ed.; 2002, Oxford University Press). Pay special attention to section 19.8. Objective: In this experiment you will carry out the reduction of a ketone to an alcohol using sodium borohydride. You will also study the stereochemistry of this reaction by reducing estrone and analyzing the products of the reaction. Scenario You are a part of an organic synthesis team at a leading drug discovery research company. In a recent meeting of division heads, the pharmacology and toxicology team proposed to carry out metabolic studies on some synthetic anabolic steroids to find drug leads that can augment breast cancer therapy. After the meeting your team has been assigned the task of synthesizing stereochemically pure steroids for this project. Your project leader has asked you to evaluate the stereochemical purity of steroids that can be obtained by utilizing various reagents for the synthesis. Steroids are a class of bioactive molecules that play a vital role in the body such as controlling meiosis, carbohydrate metabolism, fat storage, muscle growth and immune function. Several natural and synthetic steroids are used therapeutically in treating metabolic disorders, weight loss and breast cancer. Figure 3.1. Testosterone – a typical steroid. Structurally, steroids contain a skeleton of four fused rings to which various functional groups are attached. An important synthetic conversion of steroids that affects their metabolism is methylation at the 17-position of the skeleton. Methylation at this position retards significantly the metabolism of the steroid by the liver into waste products. Therefore, 17-methylated steroids usually have a longer half life than their non-methylated counterparts. The basis of the retarded metabolism is that the 17-methyl group prevents the steroid from fitting into the active site on the various liver enzymes that process steroids. For this reason, most oral steroids are methylated at 17-position. 7 Figure 3.2. Examples of orally available synthetic steroids methylated at the 17-position. In order to design an appropriate scheme to synthesize stereochemically pure steroids, your team leader has asked you to investigate the feasibility of using steroids with a carbonyl group at the 17-position as starting materials. An easy way to investigate the stereochemical course of reduction is to carry out the reduction of estrone and determine whether the reaction leads to a single stereochemically pure product or a mixture of stereoisomers. Understanding the Experiment Metal hydrides (sources of hydride anion H:–) of the group 3A elements, such as lithium aluminum hydride (LiAlH4) and sodium borohydride (NaBH4), are widely used to reduce carbonyl compounds to their corresponding alcohols. Lithium aluminum hydride reduces many compounds containing carbonyl groups – aldehydes, ketones, carboxylic acids, esters and amides. By contrast, NaBH4 is a weaker reducing agent and reduces only aldehydes and ketones. 8 The reduced reactivity of sodium borohydride and the fact that sodium ion is a weaker Lewis acid than lithium ion requires that it be used in protic solvents such as alcohol. In contrast, lithium aluminum hydride reacts rapidly with, and is thus destroyed by, protic solvents and therefore must be used under anhydrous conditions in aprotic solvents free of any carbonyl functionality. Ease of handling and optimal reactivity (and low cost) makes sodium borohydride the reagent of choice for reducing aldehydes and ketones, not just in the laboratory but in large scale industrial processes as well. In this experiment, you will carry out the reduction of estrone to estradiol using sodium borohydride in methanol as the solvent. Estrone and estradiol are classified as estrogens – steroid compounds that function as female sex hormones. They promote the development of female secondary sex characteristics, such as breasts, and are also involved in the thickening of the endometrium and other aspects of regulating the menstrual cycle. Like all steroid hormones, estrogens readily diffuse across the cell membrane; inside the cell, they interact with estrogen receptors. Estrone and estradiol were first isolated from the urine of pregnant women. Besides functioning as female sex hormones, natural and synthetic estrogens find their therapeutic applications in hormone replacement therapy and treatment of breast cancer. Q1. Examine the structures of 17-D-estradiol and 17-E-estradiol carefully and determine the stereochemical relationship between the two isomers. When estrone is treated with sodium borohydride (NaBH4), reduction of the carbonyl group takes place to yield an alcohol. That is, estrone is converted to either 17-D-estradiol or 17-E-estradiol. In order to predict the stereochemical course of the reaction, some additional structural features of the estrone molecule should be considered. A steroid with all-trans ring junctions, such as estrone, is fixed in a rigid, almost disc-like shape. The angular methyl group (see structure below) is in an axial position, and is said by convention to occupy the E (beta) face of the steroid. The other face —the “underside” of the steroid— is referred to as the D (alpha) face. When sodium borohydride attacks the carbonyl group, it must attack the carbonyl S-orbitals. In principle this can occur from either face of the steroid. From examination of the structure below, however, one can see that the two different modes of approach of the BH4í are not equivalent. 9 Q2. On the basis of the structure given above, which face of the carbonyl group in estrone will be attacked most rapidly by borohydride anion? How does this affect the orientation of the hydroxy group in the product? Q3. Predict the major diastereomer that will be formed by borohydride reduction of estrone. Procedure Add 5 mL of 95% ethanol and 40 mg (148 Pmol) of estrone into a 25-mL scintillation vial along with a magnetic stir-bar. Adjust the hot plate to the power setting “3” (temperature of heating block ~80 °C), place the reaction flask in a heating block on the hot plate and stir until estrone is completely dissolved. Solubility of estrone: One gram of estrone is soluble in 250 mL of 95% ethanol at 15°C. However it requires only 50 mL of boiling alcohol to dissolve the same amount of estrone. It will take roughly 5 minutes for estrone to be dissolved in the 95% EtOH. Obtain a silica gel TLC plate and carefully draw a line with a soft pencil 1.0 cm from the bottom of the plate to mark the origin. Delineate five separate lanes by making five small, evenly spaced marks perpendicular to the origin line; number these 1 through 5. With a capillary, remove a small sample of your estrone solution and apply it three times to LANE 1. Add 1 mL of 0.5 M ethanolic NaBH4 to the estrone solution and stir the reaction mixture for 15 minutes. Note that the initial mixture will be fairly clear and may become cloudy after a couple of minutes. At the end of this period, remove a sample of the reactions with a capillary tube and spot it in LANE 2 of the TLC plate. Adjust the power setting of the hot plate to “4” (temperature of heating block ~90 °C) and boil the reaction mixture while stirring. Turn off the hot plate after the reaction starts boiling and continue the stirring for 5 minutes. Slowly add distilled water to the reaction mixture with a Pasteur pipette. It will take roughly 10–12 mL of water to form a precipitate or cause the solution to become turbid. 10 The initial addition of water quenches the reaction. The borate esters, which are less soluble in water than they are in the 95% ethanol, will precipitate initially out of solution. However, borate esters react with water to yield alcohols and sodium borate that is soluble in water. If you add water slowly, you will be able to see that the initial precipitate re-dissolves. The water added will produce a mixed solvent for the crystallization of the product in the correct proportion. Remove the reaction vessel from the magnetic stirrer and cool it in an ice bath for 15 minutes to allow the product to crystallize. Collect the solid crystals by suction filtration on a Hirsh funnel; wash the product with 2 mL of distilled water and continue the aspiration for at least 15 minutes. Determine the weight of the dried product and then transfer a pinch of your product with a clean spatula into a small conical vial. Add 2 drops of 95% ethanol and mix the isolated product with a spatula. Remove a small sample with a capillary tube and spot onto LANE 3. Spot the standard solutions of 17-E-estradiol onto LANE 4 and 17-D-estradiol onto LANE 5 respectively. Fill the TLC developing chamber with 8 mL of developing solvent provided (acetone: dichloromethane: hexane, 22 : 50 : 28 by volume). Make sure that the level of the solvent is below the origin line on your TLC plate. After your TLC plate is developed, mark the solvent front and visualize the results by dipping the TLC plate in vanillin solution. Keep the TLC plate in the oven for 23 minutes and identify the isomer that you obtain as your product. Are the results consistent with your prediction? Calculate the percent yield of the final product. Determine the melting point of the dried product to verify the stereochemical assignment of your product. To obtain an IR spectrum, transfer your entire product after taking the melting point into a 3-mL conical vial. Add 0.5 mL of diethyl ether to it and vigorously mix the contents with your spatula. Wait for the contents to settle down. Put a drop of the supernatant ether solution on the center of the IR salt plate and allow it to evaporate. Repeat this process 4-5 times to get a homogenous film of your product on the salt plate. Analyze the IR spectra of your products and compare it with the spectrum of the starting material. Observation and Results Report the yield of estradiol isomer and identify its stereochemistry on the basis of your observed melting point and TLC. Report the Rf values of all the spots that you observe on TLC. Analyze the IR spectrum of your product. How does it differ from the spectrum of the starting material? Compound MW Mp (°C) estrone 270.38 260–2 272.39 220–3 17-D-estradiol 272.39 176–80 17-E-estradiol sodium borohydride 37.83 400(d) d=decomposes on melting Discussion Your discussion should include a detailed mechanism of reduction of estrone by sodium borohydride. The mechanism should clearly explain the stereochemical route followed in the reaction. Comment on stereospecificity of this reaction and answer the following questions. 1. Why is it necessary to use aprotic solvents while carrying out reductions with lithium aluminum hydride? By using methanol as an example of a protic solvent, write a balanced equation to show the reaction between lithium aluminum hydride and methanol. 11