Plasticity in visual perception and physiology
advertisement

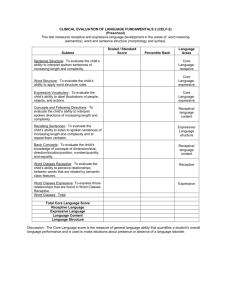
269 Plasticity in visual perception and physiology Charles D Gilbert Many factors influence our perception What of local features. we see is not strictly a reflection of the physical characteristics of a scene, on the processes but instead is highly dependent by which our brain attempts the scene. As a result, our percepts context within which local features previous visual experiences are presented, (operating of time scales), and by our expectations be before us. The substrate the lateral interactions to interpret are shaped by our over a wide range of what is likely to for these influences operating by the is found in within individual areas of the cerebral cortex and in the feedback from higher to lower order cortical areas. Address The Rockefeller University, 1230 York Avenue, New York, New York 10021-6399, USA e-mail: gilbert@rockvax.rockefeller.edu Abbreviation Vl primary visual cortex Current Opinion in Neurobiology Instead, a given input can have a very different effect depending on the other inputs that are concurrently active, and the combined effect of two inputs acting together could be very different than the sum of their individual effects. Such nonlinear behavior could be achieved by membrane properties such as voltage-dependent sodium conductances. On the other hand, alteration in a cell’s response to the same input, as a result of a previous period of conditioning, can involve an alteration in synaptic weights. The precise mechanism in which a particular pathway is altered depends partly on the time course over which the change takes places. A rapid facilitation of a pathway can be accomplished by a direct facilitation of excitatory connections or by a depression of inhibitory connections. Facilitation taking place over longer time periods can involve axonal sprouting and synaptic proliferation. Contextual 1996, 6~269-274 0 Current Biology Ltd ISSN 0959-4366 Introduction Even at early stages in the visual pathway, cells are far more flexible in their functional properties than previously thought. It had long been assumed that cells in primary visual cortex (Vl) had fixed properties, passing along the product of a stereotyped operation to the next stage in the visual pathway. Any plasticity dependent on visual experience was thought to be restricted to a period early in the life of the animal, the critical period. Furthermore, the assembly of contours and surfaces into unified percepts was assumed to take place at high levels in the visual pathway, such that the receptive fields of cells in Vl represented very small windows on the visual scene. These concepts of spatial integration and plasticity were radically modified in the past few years when it was learned that, contrary to the classical view, even at the earliest stages in the cortical processing of visual information, cells were highly mutable in their functional properties, and were capable of integrating information over a much larger part of visual space than originally believed. The modifiability of receptive field properties is referred to variously as plasticity or dynamics, but the different manifestations of cortical dynamics can involve very different mechanisms and be unrelated. For example, altering a cell’s response by changing the context does not necessarily involve a change in synaptic strengths. influences Although the receptive fields of cells may seem small when mapped with simple visual stimuli such as short line segments of a particular orientation, the way in which a cell responds to such a stimulus is dependent on the context within which that stimulus is presented. The perceptual consequences of context dependency are probably related to the process of linkage of boundary contours and fill-in of surfaces. The association of contours and surfaces belonging to a single object also requires the dissociation of these features from their background or from the contours and surfaces belonging to other objects. This is referred to as segmentation. Remarkably, the context dependency required for these processes is seen even in striate cortex. It can be observed by the facilitatory effects of a line placed outside the receptive field that, by itself, would not cause the cell to respond, but when presented in conjunction with a line segment placed within the receptive field can boost the cell’s response severalfold. This shows a dependency on relative position and orientation of the two lines, similar to psychophysical experiments. When a series of randomly placed and oriented lines are surrounding the receptive field, this will inhibit the cell’s response, but the response can be disinhibited by colinear, isoriented lines placed outside the receptive field. As a consequence, cells respond not just when the local contour is of the appropriate orientation, but also when the line segments within the global image have the appropriate geometry ([lo]; M Ito, MK Kapadia, CD Gilbert, G Westheimer, Sot NeurosGi Abstr 1995, 21:771). The phenomenon of contour saliency is illustrated in Figure 1, which shows that contours made up of similarly oriented, colinear line segments are more readily visible than ones constructed of lines with large orientation differences. This phenomenon 270 Cognitive neuroscience Figure 1 is reflected in the responses of cortical cells: even when exposed to the same stimulus within the receptive field, the cell will only respond when the local stimulus is embedded within a global pattern of linked components, obeying the same geometric constraints as perceptual saliency (Figure 1). In addition to the linkage and segmentation of contours, the process of surface fill-in may find its basis in processes occurring as early as area Vl. Perceptual fill-in has been studied with the use of artificial scotoma, a gray area within a pattern of moving lines or twinkling dots [Z], and with textured fields within a region of a contrasting texture. When a cell’s receptive field is placed within an artificial scotoma, the field is induced to expand beyond its original limits [3-6]. It has been suggested that the change is primarily an increase in responsiveness [7]. Some of the disagreement may be more a semantic one that centers on how receptive field dimensions are defined. Further experiments have shown, however, that there is a genuine increase in receptive field area (even when one normalizes for peak response), with cells responding to parts of the visual field where stimuli elicited no response before conditioning [6]. In addition to perceptual fill-in, the consequences of the expansion may be manifest as a distortion of spatial perception, such that objects lying near the boundary of the artificial scotoma appear to be pulled in towards its center [8]. This effect occurs much more rapidly than perceptual fill-in (which takes about 15 seconds), suggesting that receptive field plasticity may be observed within as little as one second. Context dependency of cortical receptive fields and its relationship to perceptual linkage. Contours placed within a noisy background of randomly placed and oriented lines are more salient (i.e. they ‘pop-out’ from a noisy background) when they are composed of similar oriented, colinear line segments (top row) than when they consist of lines of widely differing orientation (second row). The cortical substrate of this is apparent in the responses of cells in Vl : when a line segment of the appropriate orientation is placed within their receptive fields (small black squares in the four bottom frames), these cells will respond, but when the visual field surrounding the receptive field is filled with a pattern of randomly oriented and placed lines, their response is greatly suppressed (bottom half of figure, top left frame). As iso-oriented, colinear lines are progressively added outside the receptive field, however, the cells responses are greatly facilitated, thus showing a dependency of cell response on the global pattern within which local features are embedded (bottom half of figure, top left to bottom right frames). Top half of figure adapted from [441; bottom half reproduced, with permission, from (1.1. When a cell’s receptive field is located within an area of a textured pattern where there is a surrounding contrasting texture [9], the cell can respond even when the texture boundary is located well outside the classical receptive field [lo’]. The response of the cell to the texture contrast is seen not in its initial burst, but in the subsequent tonic response. Cells were capable of responding to patterns with a central area up to 12” in diameter (K Zipser, TS Lee, VAF Lamme, PH Schiller, Sod Neurosci Ah1994,20:1477). These experiments might represent a more general case of perceptual fill-in, where the central area is filled with texture rather than a uniform gray. The time course of the response did not depend on the diameter of the central area, however, which is different from the process of perceptual fill-in [ll]. It has been suggested that some of these phenomena might arise from long-range horizontal connections within Vl (for a review, see [12]). These connections were initially explored with anatomical techniques, and their functional nature has been explored further by combining optical recording with electrophysiology. These studies have confirmed the earlier findings of the relationship of intrinsic horizontal connections with cortical orientation columns. Plasticity in visual perception and physiology Gilbert One way of visualizing the functional nature of the horizontal connections is to measure the area of cortical activation resulting either from a point stimulus, known as the cortical point spread, or from a short, oriented line segment. The area of activation with a point stimulus shows a central area of spiking activity surrounded by a much larger area of subthreshold activation, with the area of spiking activity representing only 5% of the total activated area measured optically [13*,14*]. The orientation specificity of the larger area supports the idea that the lateral interactions run between columns of similar orientation specificity 114.1. Optical recording has also revealed how multiple stimuli lead to interactions at the cortical level. Line segments lying outside the classical receptive field suppress the response of cells to a line segment lying within the receptive field, and do so particularly when they are of the same orientation [13*]. These findings may reveal the mechanisms underlying the modulatory effects arising from outside the classical receptive field. The functional specificity of the horizontal connections was supported by combined optical recording and slice experiments, which show that both the excitatory and inhibitory long-range interactions are between columns of similar orientation specificity [15]. The existing framework of horizontal connections can mediate increases in effective connectivity between cortical neurons whose receptive fields are caused to expand within a cortical scotoma [S]. Thus, these connections can be boosted in strength from a subthreshold, modulatory role under some conditions to a suprathreshold, driving role under others. The mechanism underlying the alteration in effective connectivity is not known, but it could involve a potentiation of excitatory connections, or a reduction in inhibitory inputs, which would then unmask the long-range excitatory inputs [ 161. The cellular mechanisms of perceptual fill-in have been explored outside of area Vl as well. Cells in area V3 respond to an artificial scotoma within seconds, even when the classical receptive field is contained within the blank area. The time course of this response corresponds to that of the psychophysical measurements of fill-in (17.1, and the gradual build up of the response was referred to as ‘climbing activity’. The response of cells to this pattern was not observed in Vl by the DeWeerd et al. [17’] group, although, as discussed above, it was seen in the earlier studies [3,5]. The time course of receptive field expansion in Vl has not yet been measured, so it is not yet possible to determine its relationship to that of perceptual fill-in. In dealing with these phenomena, there is a potential for confusion between the meaning of receptive field expansion as measured by simple stimuli and a cell’s response to complex patterns outside the classical receptive field. The two phenomena may represent more of a continuum: initially, there are subthreshold, modulatory facilitatory influences 271 from outside the receptive field, possibly mediated by intrinsic long-range horizontal connections, which cannot be seen with a single-line stimulus, but can be seen by the conjunction of stimuli inside and outside the receptive field (such as that observed by Kapadia eta/. [I’]). As these influences are facilitated, either by direct potentiation of the horizontal connections or by unmasking via adapting out inhibition, they can be elevated to a suprathreshold influence. To a degree, the suprathreshold effects may require multiple stimuli, such as the pattern of lines used in an artificial scotoma, or, if elevated sufficiently, may even be seen with the original simple stimuli used to define the classical receptive field, such as a single line, and, therefore, may be more clearly defined as a receptive field expansion. It has been variously argued that the contextual effects involve lateral interactions at lower levels or feedback interactions from higher order cortical areas. It has been found, for example, that grouping operations are lost in animals with V2 lesions, and that this area may therefore be involved in figure-ground segregation [18]. Facilitation produced by linked contours observed in area Vl might be mediated, then, by feedback from area V2, by the horizontal connections within Vl, or by both. The characteristics of the lateral interactions of Vl, however, suggest that they are well suited to mediate these effects. An alternative hypothesis is that the feedback connections modulate the surround interactions that are specified by horizontal connections, with a joint role of the two. This idea might further explain why there would be a difference between the explicit receptive field properties seen by summation within the receptive field and the non-classical interactions from outside the receptive field. The latter would allow the non-classical properties to be more dynamic, set up by feedforward interactions mediated by horizontal connections, but gated by attentional mechanisms mediated by feedback connections. Perceptual learning Longer term plasticity of receptive field structure and functional architecture in visual cortex may underlie perceptual learning. Whereas one usually associates visual learning with acquiring the ability to recognize novel objects, which, based on neurological literature is thought to be primarily based in higher order visual cortical areas in the temporal lobe, the ability to discriminate much simpler attributes may be associated with much earlier stages in the visual pathway. Perceptual learning has been studied for over 100 years (for a review, see [19]), with improvement demonstrated with practice in various hyperacuity tasks, orientation and direction of movement discrimination. Recent studies on its specificity have suggested the involvement of Vl. Below, I summarize some of the recent work on this topic that has been published in the past few years. 272 Cognitive neuroscience Insight into the physiological mechanisms of perceptual learning comes from growing evidence that the learning is specific for the task, with a specificity suggestive of the involvement of early stages in visual processing. Attempts to perform a hyperacuity task results in substantial improvement over days and weeks. Training with lines of one orientation does not transfer to doing the task with lines of the orthogonal orientation [ZO]. On the other hand, when subjects are trained for vernier acuity and standard spatial resolution tasks, learning on one task does transfer to the other, and training in one eye transfers to the other eye [Zl]. This last study tested vernier acuity (also known as hyperacuity) and standard resolution acuity, and found that learning transfers between tasks. The findings of transfer raised the issue of whether the training involved an improvement in the ability to attend to the periphery, but other studies suggested instead a more specific change in the circuitry involved in the task itself, such as discrimination of orientation or spatial position. Consistent with earlier studies, however, learning was specific for the hemifield in which the training was done. The specificity of perceptual learning was further refined in improvement of orientation discrimination, which is specific for the trained orientation and highly specific for stimulus position, with little transfer between visual loci just a few degrees apart [Z&23]. Learning of hyperacuity also shows specificity for the orientation of the lines used in the hyperacuity task [ 19,241. The transfer between eyes suggests that this type of learning takes place perhaps after the stage where the input arrives to Vl, but the positional and orientation specificity suggests that it may be localized to Vl, where the retinotopic order and orientation tuning are most refined. One interesting feature of the perceptual learning studies is that learning is often most clearly seen between sessions, perhaps allowing for a period of rapid eye movement (REM) sleep that might be required for consolidation of the memory [23,25,26]. Cellular correlates of perceptual learning in the visual system have only begun to be explored, though the results of earlier work on the somatosensory and auditory system by Merzenich and others (outside the scope of this review) suggest that the improvements may involve a recruitment of cortical territory involved in the task. The idea that the receptive fields of cells and the functional architecture of the cortex can be modified as a result of visual experience has received considerable support from experiments involving retinal lesions. If one makes focal, bilateral retinal lesions, so as to remove functioning visual input from a restricted area of Vl, over time that area recovers visual responses, with the receptive fields of cells having shifted to positions immediately outside of the lesioned area of retina [27-301, in effect producing a remapping of the visual field on the cortical surface. These changes originate in the cortex, rather than at antecedent stages in visual processing, and probably involve the long-range horizontal connections, which appear to strengthen their influence during the period of reorganization by a process of sprouting and synaptogenesis [ 140,31*,32]. Although these changes take months to be completely developed, there are striking changes occurring even within minutes after making such lesions [Z&33]. The profound changes in cortical functional architecture that follow retinal lesions may represent the mechanisms of recovery following lesions of the CNS, but they may also reveal adaptive mechanisms associated with normal processes such as perceptual learning. Evidence for changes at various stages in the visual pathway associated with learning itself is beginning to emerge. Improvement in the discrimination of direction of motion of a random dot field has been shown to be associated with a change in the response characteristics of individual cells in area MT (a visual area in the parietal cortex specializing in movement) [34*]. Clearly, in area IT (interotemporal cortex), where it has long been thought that the representations of complex forms are stored, the functional specificities of cells reflect the images on which animals have been trained [35,36]. It is likely that the ability to store various forms of visual information, reflective of the ongoing experiences the animal receives, is a universal property of the cortex. The advantage of studying this phenomenon at early stages in the visual pathway is that one has a somewhat greater ability to understand its mechanism in terms of cortical connectivity, receptive field properties and functional architecture. Attention Another potential influence on the dynamic properties of cells at early levels in visual processing is that of attention or expectation. Although attentional effects had been seen in area V4 in earlier studies, more current work is establishing their presence as far back in the visual pathway as area Vl. In multiple visual areas, including Vl, focal attention to a stimulus can enhance a cell’s peak response, particularly when the stimulus is in the presence of multiple competing stimuli [37]. In area V2, a cell’s non-preferred stimulus will suppress its response to a preferred stimulus, but attention to the preferred stimulus will reduce the inhibitory effect of the non-preferred stimulus, and attention to the non-preferred stimulus will increase its inhibitory effect (J Reynolds, L Chelazzi, S Luck, R Desimone, Sot Neurosci A&F 1994, 20:1054). In area Vl, attention to a particular position in the visual field will increase a cell’s response to an oriented bar when the attended position is close to the cell’s receptive field (CE Connor, JL Gallant, DC Van Essen, Sot Neurosci A&F 1994,20:1054). In area MT, where cells are highly sensitive for movement of objects in a particular direction, cells will continue to respond when an object moves behind an occluder, as if the cell is signalling the ‘awareness’ of the presence of the object, even though for a period the retina receives no physical stimulus [38*]. To a degree, Plasticity in visual perception and physiology Gilbert the responses of cells in area MT require a higher level segmentation operation -when parsing the direction of movement of elements of a complex pattern, the cells have to differentiate between foreground and background, suggesting a possible influence of a calculation done in higher order cortical areas on the response properties of these cells (RO Duncan, GR Stoner, TD Albright, Sot Neurosci Abstr 1995, 2 1:664). In psychophysical experiments, discrimination thresholds of very simple attributes such as stereo acuity, line length estimation and orientation are strongly influenced by attention [39,40]. This is seen when an observer attends to a particular position in visual space as opposed to being uncertain as to which of a number of possible positions in which the stimulus may appear. Similar influence of positional certainty have been seen with respect to line orientation discrimination thresholds. Attention also has an influence on temporal certaintyknowing when a test object will appear will decrease the threshold of the discrimination relative to being uncertain as to which of five 500ms time intervals the test will appear [41]. One model of pattern recognition incorporates the idea that feedforward and feedback mechanisms influence cells at all stages in the visual pathway, and that the pathways selected for information transfer in an ascending direction are influenced by the product of calculations made in a descending direction [42]. Similar models suggest that higher order cortical areas make abstractions that, via the feedback connections, are fit to the more concrete data represented in the lower order areas-in effect, a process for checking hypotheses. The lower areas, in turn, send information concerning the errors between the data and the abstractions [43]. These ideas generally give to the lower areas a role of a high resolution buffer that can be modified or addressed by the higher areas. cortical area, and the feedback connections lower order stages in the visual pathway. References from higher to reading Papers of particular interest, published within the annual period of review, have been highlighted as: . of special interest I. . Kapadia MK, tto M, Gilbert CD, Westheimer G: Improvement in visual sensltrvlty by changes In local context: parallel studies in human observers and In Vl of alert monkeys. Neuron 1995, 15843-856. The authors report that the global characteristics of visual scenes influence the responses of cells in primary visual cortex to local features. These findings parallel those from perceptual studies on contour linkage and saliency. 2. Rarnachandran VS, Gregory TL: Perceptual filling-in of artificially Induced scotomas In human vision. Nature 1991, 350:699-702. 3. Pettet MW, Gilbert CD: Dynamic changes in receptive field size in cet primary visual cortex. Proc Nat/ Acad Sci USA 1992, 89:6366-6370. 4. Volchan E, Gilbert CD: Interocular transfer of receptive field expansion in cat visual cortex. Vision Res 1994, 35:1-6. 5. Das A, Gilbert CD: Recepttve field expansion In adult visual cortex Is linked to changes in strength of cortical connections. J Neurophysiol 1995, 74779-792. 6. Gilbert CD, Das A, lto M, Kapadia M, Westheimer G: Spatial integration and cortical dynamics. Proc Nat/ Acad Sci USA 1996, 93:615-622. 7. DeAngelis GC, Anzai A, Ohzawa I, Freeman RD: Receptive field structure in the visual cortex: does selective sttmulatlon induce plasticity? Proc Nat/ Acad Sci USA 1995, 92:9682-9686. 8. Kapadia MK, Gilbert CD, Westheimer G: A quantitative measure for short-term cortical plasticity in human vision. J Neurosci 1994, 14~451-457. 9. Knierim JJ, Van Essen DC: Neuronal responses to stattc texture patterns In area VI of the alert Macaque monkey. J NeurophysiollQQ2 67:961-980. 10. . Lamme VAF: The neurophysiology of figure-ground segregation in primary visual cortex. J Neurosci 1995, 151606-1615. Cells in the primary visual cortex respond to texture contrasts, even when the texture boundary is well outside the classical receptive field. 11. Paradiso MA, Nakayama K: Brightness Vision Res 1991, 31:1221-1236. 12. Gilbert CD: Horizontal integration and cortical dynamics. Neuron 1992, 9:1-l 3. Conclusions In contrast to the classical idea that the response properties of cells at early levels of visual processing in the adult are fixed and that these cells deal only with local features, it now appears that they are highly dynamic and perform considerable spatial integration. There are numerous influences that play a role in sculpting the functional properties of cells and the functional architecture of the cortex, including the context within which a feature is presented, the history of previous visual stimulation (operating over a range of time scales), and attention or expectation. These findings require a new way of thinking of receptive fields, such that rather than being fixed in specificity and restricted in area, fields must be thought of as adaptive filters, modified by context, experience and expectation, and sensitive to the global geometric characteristics of visual scenes. The mechanisms underlying this plasticity include feedforward processes, such as those mediated by the long-range intrinsic horizontal connections within each and recommended 273 perception and filling in. 13. . Grinvald A, Lieke EE, Frostig RD, Hildesheim R: Cortical pointspread fur&ton and long-range lateral interactions revealed by real-time optical imaging of Macaque monkey primary visual cortex. J Neurosci 1994, 14:2545-2568. Using voltage-sensitive dyes, the authors demonstrate lateral interactions in monkey primary visual cortex via optical imaging. 14. . Das A, Gilbert CD: Long-range horizontal connections and their role In cortical reorganization revealed by optical recording of cat primary visual cortex. Nature 1995, 375:780-784. In this study, the authors reveal functional interactions across primary visual cortex. These interactions possibly represent the substrate of contextual interactions seen in physiological and perceptual studies. 15. Weliky M, Kandler K, Fitzpatrick D, Katz LC: patterns of excitation and inhibition evoked by horizontal connections in visual cortex share a common relatlonship to orientation columns. Neuron 1995, 15541-552. 16. Xing J, Gerstein GL: Simulation of dynamic receptive fields in primary visual cortex. Vision Res 1994, 34:1901-l 911. 1 7. . DeWeerd P, Gattas R, Desimone R, Ungerleider LG: Responses of cells in monkey visual cortex during perceptual filling-in of an artificial scotoma. Nature 1995, 377:731-734. The authors observed changing levels of activity for cells in visual cortical area V3 during conditioning with an artificial scotoma on a time coursa similar to that seen for perceptual fill-in. 274 Cognitive neuroscience 18. Meriaan WI-I. Nealev TA. Maunsell JHR:Visual effects of lesi&s of cOrtical erea’V2 in Macaques. I Neufosci 1993, 13:3160-3191. 19. Gilbert CD: Early perceptual learnlng. Proc Nat/ Acad Sci USA 1994,Sl :1195-l 197. 20. Fahle M, Edelman S: Long-term learnlng In vernler acuity: effects of’stimulus orientation, range and of feedback. Vision Res 1993,33:397-412. 21. Beard BL, Levi DM, Reich LN: Perceptual learning in parafoveal vision. Vision Res 1995, 35:1679-l 690. 22. Karni A, Sagi D: Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proc Nat/ Acad Sci USA 1991, 88:4966-4970. 23. Schoups AA, Vogels R, Orban GA: Human perceptual learning in identifying the oblique orientation: retlnotopy, orientation specificity and monocularlty. J Physiol (Land) 1995, 33. 34. . Zohary E, Celebrini S, Britten KH, Newsome WT: Neuronal plasticity that underlies improvemerit in perceptual performance. Science 1994,283:1289-l 292. The response propertiesof cells in area MT change in a parallel relationship to the animals improvementin performinga movementdiscriminationtask. 35. Kobatake E, Tanaka K, Tamori Y: Long term learning changes the stimulus selectivity of cells In the inferotemporal cortex of adult monkeys. Neurosci Res 1992 17(suppl):S237. 36. Sakai K, MiyashitaY: Neuronal tuning to learned complex forms In vision. Neuroreport 1994, 5:829-832. 37. Matter BC: Focal attention produces spatially selective processing In visual cortical areas Vl, V2, and V4 in the presence of competing stimuli. J Neurophysioll993, 70:909-919. 483:797-810. 24. Saarinen J, Levi J: Perceptual learning in vernier acuity: what is learned? Vision Res 1995, 35:519-527. 25. Karni A, Sagi D: The time course of learning a visual skill. Nature 1993, 385:250-252. 26. Kami A, Tsnne D, Rubenstein BS, Askenasy JJM, Sagi D: Dependence on REM sleep of overnight improvement of a perceptual skill. Science 1994, 285:679-682. 27. Gilbert CD, Hirsch JA, Wiesel TN: Lateral interactions in visual cortex. Cold Spring Harb Symp Ouant Bioll990, 55:663-677. 26. Gilbert CD, Wiesel TN: Receptive field dynamics in adult primary visual cortex. Nature 1992, 358:150-l 52. Chino YM. Kass JH. Smith EL Ill. Lanaston AL. Chena H: Rapid re&ganizatibn of co&l ma?psIn adi)t c&following restricted deafferentation in retina. Vision Res 1992, 32:789-796. 38. Assad JA, Maunsell JHR: Neuronal correlates of Inferred motion in primate posterior perietal cortex. Nature 1995, 373:518-521 &Is respond to ‘movement’of occluded objects, representingthe inference of motion in the absence of a physical stimulus. 39. 40. Lindblom B, Westheimer G: Spatial uncertainty In stereoacuity tests: Implications for clinical vision test design. Acta Ophthalmol 1992, 70:60-65. LindblomB, Westheimer G: Uncertainty effects In orientation discrimination of foveally seen lines in human observers. J Physiol (Land) 1992, 45411-8. Kaas JH, KrubitzerIA, Chino YM, LangstonAL, Polley EH, Blair N: Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science 1990, 248:229-231. 41. Heinen SJ, Skavenski AA: Recovery of visual responses in fovea1 Vl neurons following bilateral fovea1 lesions in adult monkey. Exp Brain Res 1991, 83670-674. Westheimer G. Lev E: Temooral uncertaintv effects on orientation dl.&ri~inatlon’and stereoscopic thresholds. J Opt Sot Am A 1996, 13: in press. 42. Darian-SmithC, Gilbert CD: Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature 1994, 388~737-740. Synaptogenesisin the adult cortex resultingfrom alterations in visualexperience. Ullman S: Sequence seeking and counter streams: a computational model for bidirectional information flow In the visual cortex. Cereb Cortex 1995, 5:1-l 1. 43. Mumford D: On the computational architecture of the neocorten II. The role of cotico-cortical loops. Viol Cybem 1992, 68:241-251. 32. 44. Field DJ, Hayes A, Hess RF: Contour integration by the human visual system: evidence for a local “association field: Vision Res 1993, 33:173-l 93. 29. 30. 31. . Darian-SmithC, Gilbert CD: Topograpliic reorganization in the striate cortex of the adult cat and monkey is cortically mediated. J Neurosci 1995, 15:1631-l 647.