TRUMAN COLLEGE Spring 2014 "Our Mission dedicates us to
advertisement

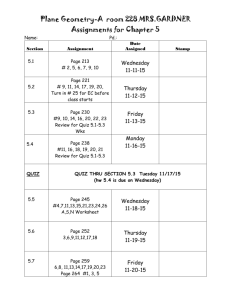
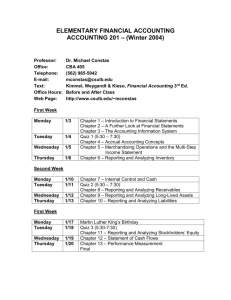
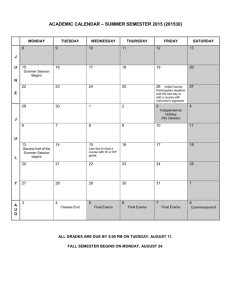
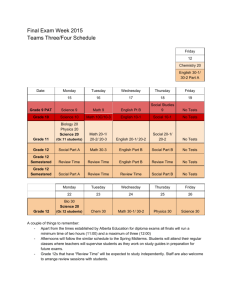
TRUMAN COLLEGE Spring 2014 "Our Mission dedicates us to deliver high-quality, innovative, affordable and accessible educational opportunities and services that prepare students for a rapidly changing and diverse global economy. 1146-TR-P-CRED-REG-CHEM-205-1-FGH-64917-SPR2014 SYLLABUS Chem. 205-1-FGH (Organic Chemistry) Studio Class, Room #3170 Tuesday, Thursday: 8:30 AM — 12:20 PM Instructor: Ahmed A. Hakeem, Contact: Room# 3840, Tel: 773-907-4076; e-mail: ahakeem@ccc.edu Office Hours: Room #3840 Required Materials: M, W 11:30 AM – 12:30 PM 3:30 PM - 5:45 PM (By Appointment: 8:45 PM – 9:15 PM) T, Th. 12:30 PM – 2:00 PM (By Appointment: 2:00 PM – 3:30 PM) Textbook: Organic Chemistry 5th Ed. (Enhanced Edition) by W. H. Brown, C. S. Foote, B. L. Iversion & E. V. Anslyn (ISBN 13- 978-0-538-49675-9) Custom Lab Manual Student Lab Notebook or similar with duplicate numbered pages Optional: Molecular Models (strongly advised), Colored pens or pencils for taking notes (three colors plus black) Course Description: Fundamentals of organic chemistry, orbitals and structural theory, aliphatic and aromatic hydrocarbons, alkyl halides, structural isomerism, introduction to functional groups, nomenclature, stereochemistry, reaction mechanisms, resonance theory, and spectroscopy. Credit hrs 6, Contact hrs 8 (4 hrs Lect. + 4 hrs Lab) Prerequisite: A grade of “C” or better in Chem. 201 & Chem. 203 or consent of the department chair. If you do not have the prerequisites, you need to see me. STUDENT LEARNING OUTCOMES FOR CHEMISTRY 205-1: Upon successful completion of this course the student will be able to: 1. Solve problems in organic chemistry using chemical concepts such as structural analysis, mechanistic theory, spectroscopic analysis, and elements of synthesis. 2. Safely handle and manipulate chemicals and standard laboratory equipment in the laboratory to achieve synthesis, purification, and analysis of organic compounds. 3. Record, graph, chart, analyze, and interpret data obtained from experimentation. 4. Integrate the knowledge of organic chemistry, including all of the above, and the role that the subject plays within the broad context of the chemical and biological sciences and the real world (living organisms, issues and challenges that confront our society, etc.). 5. Communicate an understanding of key chemical concepts, including all of the above, in oral and written form. Active Pursuit: In order for students to remain in this course, they must actively pursue the objectives for this course. At midterm, any student who does not complete and turn in all major class assignments, lab reports, essay, and quiz will receive a midterm grade of Administrative withdrawal (ADW). Failure to classroom participation will also result in ADW. An ADW grade has consequences on student’s GPA, financial aid, and other aspects of attending Truman. Simply attending the course and not producing the work and /or participating, does not constitute active pursuit. 1 Course Content: The focus of lectures, homework assignments, and problem sessions will be the material covered in Chapters 1 through 14 and Chapters 21 through 22. The course outline and lecture schedule give further details. Writing Assignments: As appropriate to the discipline are part of the course Laboratory Work: The lab component consists of approximately 10 experiments plus one take home lab (structure elucidation by spectroscopy). Check blackboard website for any changes. You need to read and be familiar with the experimental procedure(s) you will be using for your lab. Always bring a copy to the lab. NB: Prior to the scheduled lab period, students are required to have (1) carefully read the experimental write-up and (2) complete any pre-lab assignment prior to the beginning of the lab period. Pre-lab assignments are to be handed in at the beginning of the lab period. All experimental data are to be recorded in ink directly into your notebook. Do not write on pieces of scrap paper or paper towel as they tend to get lost. Lab Reports: Use word processing for all text, however, calculations and chemical equations may be handwritten. The report should be on 8 1/2 x 11 paper with no fringe edges and should have the appearance of a professional report. Pages should o be named and stapled together. Reports should be submitted one week after performing the experiment. There will be two points deduction for every class day late. No lab report will be accepted if it is more than two weeks late. Each lab report is worth 15 points. There are no make-up labs and all labs must be performed during the scheduled lab session. Your lab report should include the following: 1. Cover sheet (1 pt): The cover sheet should appear as follows Course #:_____________ Section #: _______ Name of the Experiment: _________________ Your name: _________________ Date performed: ______________ Date submitted: ______________ 2. Abstract (2 pt): A brief summary (not more than 5 sentences) of the experiment which includes what you did, the method used and a brief statement regarding your results or lack of. 3. Methods (2 pts): A narrative of the experimental procedure in your own words. Known procedures need not be rewritten. Rather the references need to be cited and any modifications done to the published method stated. 4. Results (2 pts): A brief narrative of the experimental results. 5. Discussion (2 pts): Discuss the relationship between your experimental results and the expected results. Give plausible reasons (not limited to experimental errors) for any differences. Include the relevance of the results and your experience doing this experiment in relation to the stated objectives. 6. References (1 pts): List your sources. For example: Handbook of Chemistry and Physics, the proper citation of the handbook, texts or websites. 7. Figures, Tables, Data, and Spectra (2 pts): This section includes your tabulated data and results, calculations, graphs, and spectra. 8. Answers to questions (3 pts): Pre and Post Laboratory questions Note (1): Laboratory performance points are included in the scores. Failure to follow correct laboratory techniques or laboratory safety protocols will reduce your score. Note (2): This is a lab course and as such you will need to satisfactorily complete the labs in addition to maintaining the letter grade in order to pass this course. 2 Quizzes: A quiz will be given every week, generally on Tuesdays, at the beginning of the class except if an exam is scheduled. 10 quizzes and 1 writing assignment will be given. No make-up quizzes are allowed. As a department policy, no electronic dictionaries, palm computers, cell phones are allowed during quizzes or exams. Exams: Three interim exams and one final ACS exam (total four exams). No make-up exams will be given. The approximate exam dates are given in the schedule. Evaluation: Your grade will be based on your performance in the following. (25% from each will determine the final grade). 10 Quizzes + 1 Writing Assignment (20 pts ea) 9 Labs + 1 Take home spectroscopy assignment (15 pts ea) 3 Interim Exams (100 pts ea) 1 Final Exam (ACS) (100 pts) Total 220 pts 150 pts 300 pts 100 pts 770 pts (25%) (25%) (25%) (25%) The following scale will be used to assign letter grades: A B C D F 90% and above 80%-89% 70%-79% 50%-69% <50% Homework: Doing homework is essential to success in this class. Homework will be assigned to you in the class or through the blackboard. Attendance: Your attendance is required at all classes. Absence in the first two classes will result “NSW”. Absence in the exam, quiz, or lab will score ‘0’. Excessive absence or late arrival will result in non-active pursuit of the course leading to being dropped from the course or receiving an ‘F’. It is the responsibility of the student to contact the instructor regarding missing work. There will be no make up exams, quizzes or labs. Academic Support Services: Your success in this class is important to me. The following support services are available to student at Truman College: Tutoring Center: Students are encouraged to get tutoring help with their assignments in room L129, 773- 907-4785, www.trumancollege.edu/studentservices/tutoring. Student Success and Leadership Institute (SSLI): Other support services for students to achieve educational goals. Room 1435, 773-907-4714, www.trumancollege.edu/studentservices/ssli TRIO Student Support Services: For low-income students, first generation college students, or students with disabilities who need academic support, room 1435, 773-907-4797, www.trumancollege.edu/trio. Registration is required at the start of each semester. Disability Access Center: If you have any concerns about participating or accomplishing the required course work because of a disability or medical condition, please see me after class, and also visit Ms. Linda Ford in the Student Services office. The Disability Access Center verifies needs pursuant to the American Disabilities Act (ADA), determines academic accommodations, and issues accommodation letters. Room 1428, 773-907-4725, www.trumancollege.edu/studentservices/dac. Registration is required at the start of each semester. FERPA: Family Educations Rights and Privacy Act (FERPA) is a federal law that protects the privacy of student’s educational needs. This law prohibits the faculty from revealing about students or students records over the phone or unsecure e-mail. The CCC e-mail meets FERPA requirements. www.ed.gov/policy/gen/guid/fpo/ferpa/index.html Important things to remember: 1. Be familiar with the academic integrity policy. It appears in the student handbook. Cheating of any kind or taking of anyone else’s work will not be tolerated. 2. Turn off all cell phones while in class. 3. Any assignment handed in late will have points off. (2 points daily after the due date) 3 4. Frequently check blackboard for any notices. 5. You are responsible for everything said in class even if you are not there. 6. Remember to read this syllabus (it is for your information) and refer to it from time to time. Academic Dishonesty: "Academic dishonesty” is a serious offense, which includes but is not limited to the following: Cheating, complicity, fabrication, falsification, forgery, and plagiarism. Cheating involves copying another student’s paper, exam, quiz or use of technology devices to exchange information during class time and/or testing. It also involves the unauthorized use of notes, calculators, and other devices or study aids. In addition, it also includes the unauthorized collaboration on academic work of any sort. Complicity, on the other hand, involves the attempt to assist another student to commit an act of academic dishonesty. Fabrication and falsification, respectively, involve the invention or alteration of any information (data, results, sources, identity, and so forth) in academic work. Another example of academic dishonesty is forgery, which involves the duplication of a signature in order to represent it as authentic. Lastly, plagiarism involves the failure to acknowledge sources (of ideas, facts, charges, illustrations and so forth) properly in academic work, thus falsely representing another’s ideas as one’s own." - p. 39, CCC Student Policy Manual TOPICAL OUTLINE (A): List of Lecture Topics: Chapter 1: Covalent Bonding and Shapes of Molecules Chapter 2: Alkanes and Cycloalkanes Chapter 3: Stereochemistry and Chirality Chapter 4: Acids and Bases Chapter 5: Alkenes: Bonding, Nomenclature, and Properties ---------------------------------------------------------------------------------Exam 1 Chapter 6: Reactions of Alkenes Chapter 7: Alkynes Chapter 8: Haloalkanes, Halogenations, and Radical Reactions Chapter 9: Nucleophilic Substitution and β-Elimination Chapter 10: Alcohols Chapter 11: Ethers, Epoxides, and Sulfides Chapter 21: Benzene and the Concept of Aromaticity Chapter 22: Reactions of Benzene and its Derivatives ---------------------------------------------------------------------------------Exam 2 Chapter 12: IR Spectroscopy Chapter 13: NMR Spectroscopy Chapter 14: Mass Spectrometry ---------------------------------------------------------------------------------Exam 3 (B): List of Labs/Experiments/Assignments: Lab check-in, Introduction to chemical hygiene plan (CHP), safety instructions, safety quiz, and lab tour (eyes wash stations, fire hydrants, emergency showers, waste disposal, balances, and microscale laboratory kits) Lab 1: Lab 2: Lab 3: Lab 4: Lab 5: Lab 6 Lab 7: Lab 8: Lab 9: Physical Properties of Organic Compounds (m.p; b.p; ignition test) Structure in Organic Compounds: Use of Molecular Models I Distillation (semi-micro/fractional) Stereochemistry: Use of Molecular Models II Isolation of Ingredients of an Analgesic Drug Column Chromatography Acetylsalicylic Acid Use of extraction to isolate a neutral compound from a mixture containing acid or base impurity Nitration of Methyl benzoate 4 Lab 10: (Take Home) Identification of Unknowns by IR, NMR, MS, and CHN (Take Home): Writing Assignment (Nobel laureates and their discoveries in organic chemistry) Tentative Schedule (Spring 2014) Below is a tentative schedule of lecture topics, labs, quizzes, and exams. The schedule/list of topics and lab experiments are subject to change. Students will be notified of changes, if any. Week Day Date Lecture Topic/Quiz/Exam/Lab Tuesday 1/14 Assessment Test, Introduction of the course, SLOs, goals, attendance Chapter 1: Introduction/Review of Chemical Bonding 1 2 Thursday 1/16 Chapter 2: Alkanes and Cycloalkanes Tuesday 1/21 Chapter 2 contd. Thursday 1/23 Chapter 3: Stereochemistry and Chirality Tuesday 1/28 Quiz 1 (20 min. Ch. 1) Chapter 3 contd. 3 4 Thursday 1/30 Quiz 2 (20 min. Ch. 2) Tuesday 2/4 Ch 3contd. Thursday 2/6 Lab check-in, Introduction to chemical hygiene plan (CHP), safety instructions/video clip, quiz, and lab tour (eyes wash stations, fire hydrants, emergency showers, waste disposal, balances, and microscale laboratory kits) Lab 1: Physical Properties of Organic Compounds (m.p, b.p, ignition test) Tuesday 2/11 Quiz 3 (20 min. Ch. 3) Chapter 4: Acids and Bases 5 Thursday 2/13 Ch 4 contd. Lab 2: Structure in Organic Compounds: Use of Molecular Models I Tuesday 2/18 Quiz 4 (20 min. Ch. 4) Chapter 5: Alkenes 6 Thursday 2/20 Ch 5 contd. Lab 3: Distillation (semimicro/fractional) Tuesday 2/25 Quiz 5 (20 min. Ch. 5) Chapter 6: Reactions of Alkenes 7 Thursday 2/27 Ch 6 contd. Lab 4: Stereochemistry: Use of Molecular Models II Tuesday 8 3/4 Exam 1 (1 hr. Chapters 1-5) Chapter 7: Alkynes 5 Thursday 3/6 Ch 7 contd. Lab 5: Isolation of Ingredients of an Analgesic Drug Tuesday 3/11 Quiz 6 (20 min. Ch. 6) Chapter 8: Haloalkanes, Halogenation and Radical Reactions 9 Thursday 3/13 Ch 8 contd. Lab 6: Column Chromatography Tuesday 3/18 Quiz 7: (20 min. Ch. 7) Chapter 9: Nucleophilic Substitution and Elimination 10 Thursday 3/20 Ch 9 contd. Lab 7: Acetylsalicylic Acid Tuesday 3/25 Quiz 8: (20 min. Ch. 8) Chapter 10: Alcohols 11 Thursday 3/27 Ch 10 contd. Lab 8: Use of extraction to isolate a neutral compound from a mixture containing acid or base impurity Tuesday 4/1 Quiz 9: (20 min. Ch. 9) Chapter 11: Ethers, Epoxides and Sulfides 12 Thursday 4/3 Lab 9: Nitration of Methylbenzoate Take Home Writing Assignment: Nobel Laureates and their discoveries Tuesday 4/8 Chapter 21: Benzene and the Concept of Aromaticity Chapter 22: Reactions of Benzene and its Derivatives 13 Thursday 4/10 Quiz 10: (20 min. Ch 10) Chapter 22 contd. 14 Tuesday 4/15 ---------------------Spring Break---------------------- Thursday 4/17 ----------------------Spring Break---------------------- Tuesday 4/22 Exam 2 (1 hr. Ch. 7-11 and Ch. 21 & Ch. 22) Chapter 12: IR Spectroscopy 15 Thursday 4/24 Chapter 13: NMR Spectroscopy Tuesday 4/29 Chapter 14: Mass Spectrometry Take Home Assignment : Identification of unknowns by spectroscopy (NMR, IR, MS) 16 Thursday 5/1 Exercises/Discussion: How to use spectroscopic data d for structure determination/IHD Tuesday 5/6 Exam 3 (1 hr. Ch. 12-14) 6 Review/Questions & Answers 17 Thursday 5/8 Final Exam ACS (Comprehensive/All Chapters) multiple choice Take Home Assignments due ------------------------End of Spring Semester--------------------- 7