Electron Diffraction - Faculty of Science at Bilkent University
advertisement

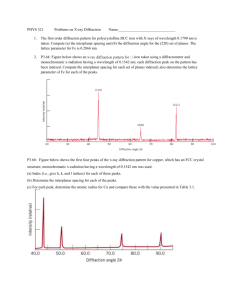
Electron Diffraction Name Lastname DD.MM.YYYY Department of Physics, Bilkent University, Bilkent, Ankara Abstract—The abstract summarizes the experiment you have performed and its major results in a few sentences. Do not go into detail here. Example: We investigated the diffraction of electrons through a polycrystalline graphite layer onto a fluorescent screen, thereby demonstrating the wavelike behavior of electrons. Using basic diffraction theory, the lattice constants of graphite are calculated and found as d1= . . . and d2= . . . which are somewhat good agreement with reference values. The reasons behind the errors are also discussed. INTRODUCTION The introduction part should be at most one page. (Figures are exception) You should state a brief overview of the background of your experiment as if a freshman level student is the reader. The equations (if any) should be derived - as in this example - and numbered and satated in the introduction. You should give reference to the equations in later parts of the report. Do not write the same equation over and over again. Write the equation by yourself with and equation editor. In 1914, Lois De Broglie developed the theory on the wave-like properties of particles which relates the momentum and wavelength of a particle (1) h p, The constructive diffraction of waves reflected from adjacent layers of a single crystal are given by Bragg condition, (4) 2d sin n where d is lattice spacing, θ is incidence angle, and n is the order of diffraction. For polycrystalline materials, reflected beam is spread out in the shape of a cone and forms the refraction pattern. Geometrically, (5) sin 2 r R where α is the angle of deviation and R is the radius of the glass bulb. The angle of deviation is twice as the Bragg angle θ. Also by considering small angle approximations, we get the following formula. (6) sin sin 2 2 sin Using (3-6) the relation between Ua and θ is given by nh 2d sin 2MeVa 0.5 34 where h=6.626. 10 Js is the Planck’s constant. [reference] In this experiment, we apply this theory to electrons to investigate their wave-like behavior. For this purpose, we study the diffraction of accelerated electrons through a polycrystalline graphite sample, since diffraction is a wave-like phenomenon. The experiment setup consists of an electron diffraction tube and high voltage power supplies as shown in Fig. 1 Under an accelerating potential Ua, the electrons gain a kinetic energy given by p2 eV a 2M (2) 19 31 where, e=1.602.10 C and M=9.109.10 kg. Using Eq.s (1) and (2) the wavelength can be found as h 2MeU a 0.5 1500kV Ua 0.5 pm (3) (7) Referring to (7) and the radius of our glass bulb used in the experiment which is 63.5 mm we find the following formula for the lattice constants. d1, 2 1500kV V a 0.5 1 pm 2 sin 1, 2 (8) This is the most important part of the report presenting what you have done. Give your findings, perform the analysis as described in your manual (and procedure part of the preliminary work), plot figures, and make calculations. If you are asked to plot some of your data then do not present it in a table. Important note about figure size: If the features in the figure get too small when sized to report format, then plot the figures on a separate page in an adequate size. Here is an example Fig. 1. A photo of the experimental setup. Figure caption should be placed under the figure. EXPERIMENT Describe what you specifically did while you were performing the experiment. The diameters were measured by a plastic ruler several times from inner to outer boundaries and outer to inner boundaries through the center. Then the average of these measurements was taken as data which is shown on Table 1 and Fig 3. the two radii are because of the two lattice constants of graphite which are 211pm and 126pm. The experimental setup is shown on Fig.2. Here the ports G1 , G2 , G4 used for aligning the electrons G3 is used for accelerating the electrons. A grid voltage with a range of 2.5-10 kV was applied for acceleration, the Wehnelt cylinder potential, G1 arranged to -25V, G 2 to 250V and G 4 to 250 V. After turning on the power supplies, the electrons were emitted from indirectly heated surface and accelerated through the grid and hit the polycrystalline graphite then diffracted. The diffraction pattern can be observed on the fluorescent surface on the tube. By measuring the diameter of the circles on the diffraction pattern and using Ua, the lattice parameters can be calculated using (7). Table 1. Table captions are placed at the top of the table Ua (kV) 2 2.5 3.0 d1 (mm) θ1 d2 (mm) θ2 0.10 0.09 2 0.08 (rad) into a uniform beam and 1 0.07 0.06 0.05 0.04 0.03 3 4 5 6 7 8 9 10 11 Va (kV) Fig. 3. Bragg diffraction angle as a function of accelerating potential. Figure fonts should be as large as they easily be read. You can use font size of 36 for tick labels and size 48 for axes titles in origin. Fig. 2. Schematic of the experimental setup and Bragg diffraction angle, α. ANALYSIS Using equation 8, we find that d1 219 18 pm and d 2 116 3 pm. The calculated lattice constants are plotted in Fig.4 diffraction by electrons can be utilized to infer the crystal structure of materials. We applied this technique to obtain the lattice spacings of graphite and found d1 and d2 as 219±18 pm and 116±3 pm. Our results are in somewhat good agreement with reference values given. We discussed the possible reasons for the errors. (refer to your analysis’ as such. Do not repeat same things here) Figure 4: This figure represents what you should do as an example. It clearly illustrates the results and the calculations - which you should do - in one figure. As you can see data for d1 is not very consistent. Even so you should plot it. ERROR ANALYSIS: (title is not necessary) Find exact values of d1 and d2. (You have already done it in the preliminary in this case). Calculate a simple error percentage. Then comment on the result. (THIS PART IS LEFT INTENTIONALLY BLANK.You should further extend conclusion here) The electron diffraction is a well established as a characterization tool in many branches of science such as solid state physics in crystallography, and in biology for structural analysis of organic substance (proteins, bacteria etc.) REFERENCES Give all your references which you addressed in the experiment. It is very easy to find your sources via google. Also refer your references in the text. Use the format below. For example in this report exact value of d1 is stated as 211pm and is found as 219±18 pm. This concludes the good agreement. And yet is still has some error around 4%. Comment on this. For the data d2 we have more severe error. First of all exact d2 does not fall in the range of found d2. Also the error is about 9%. Comment on this result also. Even the error amount seems small this is not a good agreement. Compare it with d 1 what went wrong in this case. Remember here you are not expected to get the Nobel Prize. You will not be grades by the amount of your error. Grading is done by your level of understanding of your data. Therefore good explanation of a huge error may get the higher credit whereas a poor explanation of a good result may get lower credit. (THIS PART IS LEFT INTENTIONALLY BLANK: You should discuss your results, observations regarding your experiment, and comment on figures.) Give the answers of the questions here not in the conclusion. CONCLUSION We confirm that electrons have a wave-particle duality in their nature and can be diffracted as light in slit-like conditions (e.g. a crystal). The ability of [1] Journal Reference format is: Author(s) Name, Publication Title, Journal Title, Volume, Page Number, (Year). [2] Web Reference format is: http://www... (full address/path) [2] Electron Diffraction Experiment User Manual, PHYWE. [6] S Subramaniam, M Gerstein, D Oesterhelt, and R Henderson (1993). "Electron diffraction analysis of structural changes in the photocycle of bacteriorhodopsin," EMBO Journal 12: 1-8 (2000).