UV-Vis spectroscopy - The van Bokhoven Group
advertisement

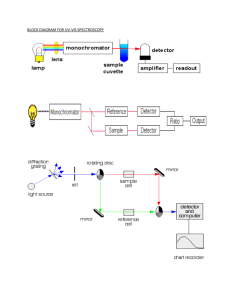
UV-Vis spectroscopy Basic theory Dr. Davide Ferri Empa, Lab. for Solid State Chemistry and Catalysis 044 823 46 09 davide.ferri@empa.ch Importance of UV-Vis in catalysis IR Raman NMR XAFS UV-Vis EPR 0 200 400 600 800 Number of publications 1000 1200 Number of publications containing in situ, catalysis, and respective method Source: ISI Web of Knowledge (Sept. 2008) The electromagnetic spectrum VISIBLE 1015-1016 Hz ULTRAVIOLET 1016-1017 Hz 200 source: Andor.com The ‘energy’ unit Typically, the wavelength (nm) is used the distance over which the wave's shape repeats x nm = 10’000’000 / x cm–1 y cm–1 = 10’000’000 / y nm Is UV-vis spectroscopy popular? pros economic non-invasive (fiber optics allowed) versatile (e.g. solid, liquid, gas) extremely sensitive (concentration) cons Broad signals (resolution) Time resolution (S/N) What is UV-vis spectroscopy? Use of ultraviolet and visible radiation Electron excitation to excited electronic level (electronic transitions) Identifies functional groups (-(C=C)n-, -C=O, -C=N, etc.) Access to molecular structure and oxidation state Electronic transitions hν Organic molecule Organic molecule anti-bonding σ* anti-bonding empty π* lone pairs e occupied n e e e π e bonding e bonding e σ n→π* n→σ* π→π* σ→σ* E= hν high e- jump → high E high E → high ν e n→π* n→σ* π→π* σ→σ* λ= c/ν high ν → low λ Electronic transitions anti-bonding σ* π* e n e π e bonding e σ n→π* n→σ* π→π* σ→σ* σ→σ* high E, low λ (<200 nm) n→σ* 150-250 nm, weak n→π* 200-700 nm, weak Condition to absorb light (200-800 nm): π and/or n orbitals π→π* 200-700 nm, intense CHROMOPHORE The UV spectrum λmax 1.0 σ* n e π σ π→π* 0.8 absorbance π* 217 nm 0.6 0.4 0.2 no visible light absorption 0.0 200 energy signal envelope E* 220 240 280 300 wavelength (nm) vibrational electronic levels rotational electronic levels E0 260 How many signals do you expect from CH3-CH=O? The UV spectrum σ* 0.10 π* 0.08 e n (O) e π σ n→π* π→π* absorbance O 0.06 0.04 0.02 no visible light absorption 0.0 200 220 240 260 wavelength (nm) 280 300 The UV spectrum Conjugation effect λmax delocalisation λ ν 171 217 258 C6H8 C4H6 C2H4 π* e π e e E The UV spectrum Conjugation effect: β-carotene white light absorbance 300 360 420 wavelength (nm) 480 540 The UV spectrum Complementary colours 650 - 780 580 - 595 500 - 560 380 - 435 435 - 480 480 - 490 If a colour is absorbed by white light, what the eye detects by mixing all other wavelengths is its complementary colour Inorganic compounds UV-vis spectra of transition metal complexes originate from Electronic d-d transitions dσ eg degenerate d-orbitals ∆ + ligand TM t2g TM dπ … Inorganic compounds dx2-y2 Crystal field theory (CFT) - electrostatic model same electronic structure of central ion as in isolated ion perturbation only by negative charges of ligand dx2-y2, dz2 dxy, dxz, dyz ∆ dz2 atom in spherical field ∆ dx2-y2 ∆ dxy dx2-y2, dz2 dxy gaseous atom dxy, dxz, dyz dyz, dxz ∆ = crystal field splitting tetrahedric field octahedric field tetragonal field dz2 square planar field Inorganic compounds d-d transitions: Cu(H2O)62+ eg degenerate d-orbitals ∆ + 6H2O light Cu2+ t2g Cu(H2O)62+ Yellow light is absorbed and the Cu2+ solution is coloured in blue (ca. 800 nm) The greater ∆, the greater the E needed to promote the e-, and the shorter λ ∆ depends on the nature of ligand, ∆NH3 > ∆H2O Inorganic compounds TM(H2O)6n+ elec. config. TM gas complex t2g1 t2g1 t2g3 t2g3eg1 t2g3eg2 Ti(H2O)63+ Ti(H2O)63+ Cr(H2O)63+ Cr(H2O)62+ Mn(H2O)62+ t2g6eg3 Cu(H2O)62+ Ni2+ absorbance 3d1 3d2 3d3 3d4 3d5 3d6 3d7 3d8 3d9 Fe2+ Cu2+ Co2+ Cr3+ Ti3+ 400 500 V4+ 600 700 800 wavelengths (nm) d-d transitions: εmax = 1 - 100 Lmol-1cm-1, weak 900 1000 Inorganic compounds d-d transitions: factors governing magnitude of ∆ Oxidation state of metal ion ∆ increases with increasing ionic charge on metal ion Nature of metal ion ∆ increases in the order 3d < 4d < 5d Number and geometry of ligands ∆ for tetrahedral complexes is larger than for octahedral ones Nature of ligands spectrochemical series I- < Br- < S2- < SCN- < Cl- < NO3- < N3- < F- < OH- < C2O42- < H2O < NCS- < CH3CN < py < NH3 < en < bipy < phen < NO2- < PPh3 < CN- < CO Inorganic compounds d-d transitions: selection rules spin rule: ∆S = 0 on promotion, no change of spin Laporte‘s rule: ∆l = ±1 d-d transition of complexes with center of simmetry are forbidden Because of selection rules, colours are faint (ε= 20 Lmol-1cm-1). Inorganic compounds UV-vis spectra of transition metal complexes originate from Electronic d-d transitions eg degenerate d-orbitals ∆ + ligand TM t2g TM Charge transfer Inorganic compounds Charge transfer complex no selection rules → intense colours (ε=50‘000 Lmol-1cm-1, strong) Association of 2 or more molecules in which a fraction of electronic charge is transferred between the molecular entities. The resulting electrostatic attraction provides a stabilizing force for the molecular complex Electron donor: source molecule Electron acceptor: receiving species CT much weaker than covalent forces Ligand field theory (LFT), based on MO Metal-to-ligand transfer (MLCT) Ligand-to-metal transfer (LMCT) Inorganic compounds Ligand field theory (LFT) involves AO of metal and ligand, therefore MO what CFT indicates as possible electronic transitions (t2g→eg) are now: πd→σdz2* or πd→ σdx2-y2* πpx*, πpy*, πpz* σp* 4p σs * 4s σd* eg ∆ t2g 3d πdxy, πdxz, πdxy σd AOL AOTM ∆ = crystal field splitting 2s σp σs MO(TML6n+) Inorganic compounds Ligand field theory (LFT) LMCT ligand with high energy lone pair or, metal with low lying empty orbitals high oxidation state (laso d0) M-L strengthened 4σ 2π∗ O 1π MLCT ligands with low lying π* orbitals (CO, CN-, SCN-) low oxidation state (high energy d orbitals) M-L strengthened, π bond of L weakened 2π∗ 2π∗ 1π 3σ C 5σ 2π∗ Metal back donation!!! CO adsorption on precious metals UV-Vis spectroscopy Instrumentation Examples for catalysis Dr. Davide Ferri Empa, Lab. for Solid State Chemistry and Catalysis 044 823 46 09 davide.ferri@empa.ch Instrumentation Dispersive instruments Measurement geometry: - transmission - diffuse reflectance double beam spectrometer single beam spectrometer In situ instrumentation Diffuse reflectance (DRUV) Fiber optics to detector gas outlet - time resolution (CCD camera) [spectra colleted at once] - coupling to reactors - 20% of light is collected - gas flows, pressure, vacuum - long meas. time - no NIR (no optical fiber > 1100 nm) - long term reproducibility (single beam) - Limited high temperature (ca. 600°C) - spectral collection (λ after λ) → different parts of spectrum do not represent same reaction time!!! Weckhuysen, Chem. Commun. (2002) 97 In situ instrumentation Integration sphere White coated integration sphere (MgO, BaSO4, Spectralon®) integration sphere - > 95% light is collected - high reflectivity - wide range of λ - only homemade cells for example, for cat. synthesis Weckhuysen, Chem. Commun. (2002) 97 Examples Determination of oxidation state: 0.1 wt% Crn+/Al2O3 Cr6+ (250, 370 nm) reduction in CO atmosphere Cr3+/Cr2+ Weckhuysen et al., Catal. Today 49 (1999) 441 Examples Determination of oxidation state: 0.1 wt% Crn+/Al2O3 calibration distribution of Crn+ Cr3+ 100 Cr6+ % Cr6+ Cr5+ Cr3+ Cr2+ 50 deconvolution 0 A B C A: calc. 550°C B: red. 200°C C: red. 300°C D: red. 400°C E: red. 600°C F: re-calc. 550°C Weckhuysen et al., Catal. Today 49 (1999) 441 D E F Examples Determination of oxidation state: 0.2 wt% Crn+/SiO2 chemometrics * PCA, principal component analysis FA, factor analysis PLS, partial least squares own routines… * decomposition into pure components (including noise) Weckhuysen et al., Catal. Today 49 (1999) 441 Examples Determination of oxidation state: 0.5 wt% Crn+/SiO2 pure components DRUV, 350°C, 2% isobutane-N 2 360 625 450 Weckhuysen et al., Catal. Today 49 (1999) 441 360 625 Examples Determination of oxidation state: 4 wt% Crn+/Al2O3 calc. 850°C, O 2 He C3H8, 21 sec C3H8, 6 min in situ DRS 20 scans, 50 msec ex situ DRS K-M intensity Cr6+ Cr3+ hydrated calcined 550°C reduced 550°C 26000 Cr6+, 26000 cm-1 C3H8 feed 40000 30000 20000 10000 wavenumber (cm-1) O2 regeneration Puurunen et al., J. Catal. 210 (2002) 418 Examples Comparison of techniques: x wt% Crn+/support HAADF-STEM xCr-Al2O3 Raman wt.% 10 5 5Cr-SBA15 1Cr-SBA15 1 0.5 1Cr-Al2O3 5Cr-Al2O3 5Cr-SBA15 0 2 4 6 8 energy (KeV) XRD 5Cr-Al2O3 10 2 4 6 8 energy (KeV) Santhosh Kumar et al., J. Catal. 261 (2009) 116 10 xCr-Al2O3 Examples Reactivity of V/TiO2 after oxidative treatment V5+Ox VxOy VxOy air flow @ 450°C O2/C3H8 @ 20°C @ 100°C @ 150°C @ 200°C V5+ → V4+ O O O V O O TiO2: 402 cm-1 V2O5: 996 cm-1 Brückner et al., Catal. Today 113 (2006) 16 Examples Reactivity of V/TiO2 after oxidative treatment UV-vis: V5+ CT (UV) V4+ d-d transitions (vis) V5+ → V4+ d-d CT Brückner et al., Catal. Today 113 (2006) 16 Examples Determination of speciation: Fe species in Fe-ZSM5 hydrated samples isolated Fe3+ oligomeric FexOy extended F2O3-like clusters calcination @ 600°C α-Fe2O3 calcined hydrated Santhosh Kumar et al., J. Catal. 227 (2004) 384