Review list of applicable procedures
The Applicable Procedures List includes surgical procedures
which have demonstrated benefit from PGDT implementation.
Review this list to help define the scope of PGDT
implementation.
Review list of applicable procedures.
Consider extending PGDT implementation to include other
medium or high risk surgical procedures.
Review the Sample Procedure Lists to see where other
institutions have applied PGDT.
Note: These lists are intended as guides for PGDT
consideration. The decision to apply PGDT should be based
on clinical evaluation by the healthcare provider.
Owner: Clinical Champion
Tools
• Applicable Procedures List
• Sample Procedure Lists
Consider patient risk profile
Owner: Clinical Champion
Surgical risk is related to both procedural and patient risk.
Intraoperative hemodynamic optimization is most impactful for
medium to high-risk surgeries.
Patient risk assessment can be based on ASA score,
PPOSUM score, or other surgical score tools.
High Risk
Predefined population who are currently “expected” to develop
significant post-operative clinical issues leading to prolonged
stay.
“At Risk”
Patients who “could” develop post operative complications.
Consider applying PGDT for surgical patients with ASA risk
score 2b plus one comorbidity or higher.
Note: The decision to apply PGDT should be based on clinical
evaluation by the healthcare provider.
ASA Patient Risk Score:
1a: Normal healthy patient
1b: Patient with mild systemic disease
1b: Normal healthy patient with anesthetic or operative risk
2a: Patient with moderate systemic disease.
2a: Patient with mild systemic disease with anesthetic or
operative risk
2b: Patient with moderate to severe systemic disease that
does not limit activity
2b: Patient with moderate systemic disease with anesthetic or
operative risk
3: Patient with severe systemic disease that is not
incapacitating
4: Patient with incapacitating disease that is a constant threat
to life
5: A moribund patient who is not expected to live 24 hours with
or without surgery
Owner: Cinical Champion
Select target population
Identify which procedures or patients are in-scope for PGDT
implementation.
1
Gather morbidity and length of stay (LOS) data for target
population
Identify a reliable data source for hospital metrics, and
understand the available data reporting capabilities. Typically,
the hospital’s Quality Officer can obtain required data.
Gather data and document current state metrics for selected
target population, including the following:
Key metrics
1. Morbidity
2. Length of stay
3. Variable cost per case
Other metrics to consider tracking
Mortality
30-day readmission rate
Patient satisfaction score
Return on investment
Specifying the types of complications to measure before and
after PGDT implementation can help to ensure a direct
comparison.
Consider whether the hospital tracks and can report on the
following measures:
•
•
•
•
•
•
•
•
•
•
•
•
Post-Op Mechanical Ventilation > 24h
Hypotension requiring pharmacological treatment
Cardiac arrhythmia requiring pharmacological treatment
Wound infection
Abdominal infection (GI surgery)
Urinary tract infection
Bacterial pneumonia
Deep Vein Thrombosis (DVT)
Pulmonary embolism
Pulmonary edema
Myocardial infarction
Cardiac arrest (exclusive of fatal outcome)
2
Owner: Clinical Champion, Quality
Tools
• Hospital Data Form
Gather morbidity and length of stay (LOS) data for target
population (continued)
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
Postoperative delirium
Stroke
Renal failure requiring dialysis
Upper gastro-intestinal bleed
Anastomotic leak (GI surgery)
Paralytic ileus
Length of stay
ICU Admission
Duration of ICU stay (if ICU admission)
Post-operative mechanical ventilation
Lactate at end of surgery or at ICU admission
Maximum ICU lactate
Re-intervention
Maximum ICU Sequential Organ Failure Assessment
(SOFA) score (or any other organ failure score)
Cumulative ICU Therapeutic Intervention Scoring System
(TISS) score (or any other resource utilization score)
Owner: Clinical Champion, Quality
Tools
• Hospital Data Form
Note: Please follow hospital guidelines on patient protected health
information. Prior to sharing hospital confidential information with
Edwards Lifesciences, please complete a non-disclosure
agreement.
Compare to benchmarks
Owner: Clinical Champion, Quality
Leverage reported data from outside sources on national
averages to understand how the hospital compares with regard
to morbidity rates and length of stay.
Identify specific opportunities for improvement
When comparing hospital data to benchmarks, identify specific
procedure types where the hospital ranks worse than preferred
state (ex. worse than national average). Consider focusing on
these opportunities for setting and measuring outcomes against
program goals.
3
Owner: Clinical Champion, Quality
Define program goals for PGDT outcomes
Owner: Clinical Champion; Quality
Input hospital data into the PGDT Benefit Estimator to calculate
potential improvement in clinical and economic outcomes based
on results from published literature.
Define project goals and timing based on estimated clinical and
economic benefits and expected compliance levels.
Tools
• PGDT Benefit Estimator
• Business Case Template
Tips for defining program goals:
Set measurable program goals at the start of the project
Establish achievable targets using documented baseline
data and published study results
Align program goals with organizational priorities
Build stakeholder alignment with program goals
Measure progress to goals periodically
Document quantifiable goals and economic benefits in a
business case.
Estimate clinical and economic benefit
Owner: Clinical Champion
Input hospital data into the PGDT Benefit Estimator to estimate
potential improvement in clinical and economic outcomes from
PGDT implementation based on results from published metaanalyses.
Document quantifiable goals and economic benefits in a
business case.
4
Tools
• PGDT Benefits Estimator
• Business Case Template
• PGDT Literature
Define investment need
Owner: Clinical Champion
Determine required investment, including monitoring equipment
(capital and disposables), team member time, and other
resources.
Monitoring equipment needs can be estimated using the
following assumptions:
•
1 disposable per procedure
•
1 monitor per Operating Room (for target population) or 1
monitor per 5 disposables per month
Tools
• PGDT Benefits Estimator
• Business Case Template
Document quantifiable investment requirement in a business
case.
Develop and communicate business case
Owner: Clinical Champion; Executive
Sponsor
Complete business case to estimate return on investment from
benefits of implementing PGDT.
Communicate the business case including clinical and
economic implications to an authorized approver or executive
sponsor. Confirm executive sponsor commitment to implement
PGDT.
5
Tools
• Business Case Template
• Sample Business Case
Identify and align core team members
Owner: Clinical Champion; Core Team
Build a cross-functional, action-oriented core team. Identify
team members who can support the project needs.
Engage a project coordinator to drive progress.
What makes a strong project coordinator?
Able to dedicate time to the project
Motivated by project participation and leadership role
Organized and well-connected within the hospital
Tools
• Project Charter Template
• Team Alignment Presentation
Educate and align the core team to the benefits of PGDT and
the program goals using the Team Alignment Presentation.
Complete business case model to estimate return on
investment from benefits of implementing PGDT.
Communicate the business case including clinical and
economic implications to an authorized approver or executive
sponsor. Confirm executive sponsor commitment to implement
PGDT.
Complete project charter
Owner: Project Coordinator or Core
Team
Completing a Project Charter helps to define program goals and
can be used to build stakeholder alignment with goals and
action plans. The Project Charter Template provides a
suggestion of content to include.
6
Tools
• Project Charter Template
Develop project plan and schedule meetings
Owner: Project Coordinator
Define a high-level action plan and timeline for program
development and implementation.
Schedule regular team meetings (minimum monthly) for at least
six months. Invite core team members to attend meetings as
required, based on project plan milestones.
Tools
• Project Charter Template
Closely manage meeting agendas and deliverables to drive
program progress.
Communicate plan to key stakeholders
Owner: Clinical Champion or
Executive Sponsor
Communicate early and often to notify impacted persons of
upcoming changes, potential benefits, and expectations.
Use the Communication Plan Template to define appropriate
messaging, target audience, and timing for program
communications.
7
Tools
• Communication Plan Template
Review and select PGDT protocols
Owner: Clinical Champion; Clinical
Leads
The Protocol Summaries describe multiple PGDT clinical
pathways which have demonstrated benefit. Review protocol
options and discuss pros and cons of each related to the target
population.
Identify a PGDT protocol to apply across the target population.
Document protocol selection in the Project Charter, and add to
standard operating procedures (SOPs). Initiate protocolspecific training with key users.
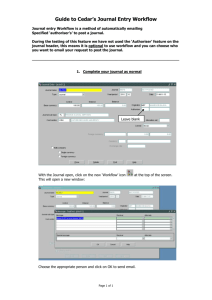
Map current workflow
Tools
• PGDT Protocol Summary
• ESA PGDT Protocol Summary
• Project Charter Template
Owner: Core Team
Workflow mapping helps to create a common vision, uncover
variations, and identify areas for process improvement. Use
standard flow chart mapping techniques to illustrate workflow,
interactions, decisions, and handoffs.
Trial the process flow by walking through it in real time. Revise
the process map as needed to accurately reflect current state.
Process Mapping:
•
Clearly define process boundaries
•
Identify first and last steps
•
Define each step – be accurate and honest
•
Make sure every loop has an escape.
Gather process information through core team experience,
observation, conversation, interviews, and research.
Note: In most processes:
– Few people have seen the total process and fully
understand the process
8
Tools
• Sample Workflow Map
Map current workflow continued
–
–
–
–
–
–
–
Owner: Core Team
Departments are managed, processes are often
unmanaged
Those that designed the process are typically no
longer there
Work is being done that adds no value to customers
Work the customer needs isn’t being done
Rework is built into the process
Inefficiencies are built into the process
Workarounds have been developed that make the
process appear to be working better than it really is
Define and build PGDT trigger(s) or checklist(s)
Checklists and other triggers help to identify appropriate
candidates for hemodynamic optimization using Perioperative
Goal-Directed Therapy, and should be built into standard
operating procedures, existing workflows, and information
systems.
Establish multiple triggers – both electronic and paper – to
maximize candidate identification and to alert key stakeholders to
prepare accordingly (surgeon, anesthesiologist, CRNA,
anesthesia tech, OR staff, scheduling, etc).
Document PGDT triggers in Project Charter Template.
Triggers can be based on surgical procedure type and patient
risk assessment (ASA score, PPOSUM, surgical score tools).
Consider including triggers in the following workflow steps:
– Scheduling
•
Alert in scheduling software based on surgical
procedure
•
Print notification on daily schedule board with attached
protocol
– Pre-screen
•
Surgical pre-screen prior to surgery
•
At Risk screening tool
– Registration / Admission
•
In-patient registration
•
Out-patient registration
•
Emergency Room transfer
– Pre-Op
•
Anesthesia pre-op assessment on day of surgery
•
Pre-op nurse screening in OR
9
Tools
• Sample Workflow Map
Owner: Core Team
Tools
• Sample Workflow Map
Define and build PGDT trigger(s) or checklist(s) (continued)
–
Owner: Core Team
Tools
•
At Risk screening tool
Intra-op
•
Surgical Time Out
•
Physician Preference Card
• Sample Workflow Map
Screening Trigger Example
Is the patient undergoing one or more of the identified
procedures?
Yes, PGDT fluid optimization should be considered to
improve post-operative outcomes [next question]
No, [next question]
Does the patient have a risk factor equivalent ASA2b with 1+ comorbidity or higher?
Yes, PGDT fluid optimization should be considered to
improve post-operative outcomes [next question]
No, [next question]
Will the patient receive perioperative goal directed therapy for
hemodynamic optimization using [the selected protocol]?
Yes, Chart on patient record
No, Chart reason for declining PGDT on patient record
Map and test new PGDT workflow with trigger(s), and
handoffs
Develop process map for the proposed PGDT workflow
incorporating trigger(s), new tasks and handoffs needed to
apply PGDT to procedures.
Physically walk through the new PGDT workflow. Identify any
gaps or obstacles, and make necessary modifications.
10
Owner: Core Team
Designate monitoring platform(s) for target population
Base monitoring platform designation on patient risk profile.
A-line based monitoring can be utilized when arterial line
placement is necessary, while non-invasive monitoring can be
used when no arterial line is needed.
Document platform designations for training purposes.
11
Owner: Clinical Champion; Clinical
Leads
Tools
• Hemodynamic Product
Information Website
• Right Patient Right Device Sheet
Communicate PGDT plan to organization
Owner: Clinical Champion
Communicate the plan and any changes in workflow to affected
stakeholders. Include in your communications plan any
clinician, administrator, or staff member who is part of or
impacted by the PGDT process. Leverage several types of
communications including posters, emails, meeting
announcements and training courses, to deliver these
messages.
Tools
• Communications Plan Template
A Communication Plan Template can help identify appropriate
messages, audience, and timing.
Develop comprehensive training plan
Owner: Clinical Champion
Training for this new PGDT workflow should be inclusive of the
following:
Benefits of PGDT: reason for implementing
Project scope: Selected procedures for PGDT
application and expectations of perioperative team
PGDT protocol: how to optimize fluid using the
selected clinical pathway
Hemodynamic monitoring platform: how to use
technology and dynamic parameters to apply a PGDT
protocol
PGDT workflow: changes to current workflow, triggers,
and handoffs
Compliance: the importance of compliance and ways
to monitor adherence to a PGDT protocol.
12
Tools
• Team Alignment Presentation
• ECCE Resources
• Hemodynamic Optimization
Modules and Presentations
Train super users, end users, and other stakeholders
Deliver a comprehensive training program. Identify super users
or educators to expand training beyond the core team and to
future impacted stakeholders.
Visit www.edwards.com/ecce and
http://www.youtube.com/ecce4you for educational resources.
Owner: Clinical Champion
Tools
• Edwards.com/ecce
• YouTube Channel
• PGDT Learning Modules
Edwards Lifesciences representatives can provide education on
PGDT and the Edwards portfolio of advanced hemodynamic
monitoring technologies.
Evaluate competencies
Owner: Educator; Super Users
Ensure adequate comprehension and competency by
establishing knowledge checks and skill tests for impacted
stakeholders.
13
Launch PGDT trigger(s), checklist(s), and add to SOP
Owner: Core Team
This is the time to “go live” with the new PGDT workflow,
triggers, and handoffs. During this activation period, triggers
should clearly identify the target population, and users should
apply the selected PGDT protocol to optimize perioperative fluid
status.
Evaluate workflow, trigger(s), and handoffs
Owner: Core Team
Closely monitor and evaluate the new PGDT workflow, triggers,
and handoffs to ensure a smooth transition and to identify any
remaining gaps.
Adjust workflow as needed
Owner: Core Team
If gaps are identified, make slight modifications to the PGDT
workflow, triggers, and handoffs as needed. Retrain and
clearly communicate any changes to impacted stakeholders.
14
Develop method to track compliance for PGDT application
Owner: Core Team
and protocol
Compliance is key to the successful implementation of any
protocol. The purpose of implementing protocols in healthcare is
to improve safety, enhance quality of care, and reduce variation
that can occur in clinical practice. Lack of compliance with the
protocol can impede progress to these goals.
Three types of compliance to monitor:
1. Compliance to procedure type
Leverage triggers and patient EMR to document and
track PGDT application to appropriate patients.
2. Compliance to protocol
Use Case Reports generated from monitor data or the
Compliance Tracking Worksheet to assess practice
consistency with protocol.
3. Compliance to tracking
Evaluate completeness of compliance data to
understand potential impact on outcome.
Track and document compliance levels
Tools
• Monitor Data Download
Instructions
• Case Report Generator
• Compliance Tracking Worksheet
Owner: Core Team
Identify a clinical resource to regularly monitor compliance to
targeted procedure type and compliance to the selected
protocol.
15
Gather morbidity, length of stay (LOS), and PGDT
Owner: Core Team
compliance data for target population
Gather data and document post-PGDT metrics for selected
target population, using the same metrics documented in the
current state analysis. Specifically, include key metrics such as
morbidity, length of stay, and variable cost per case. Ensure a
direct comparison by measuring the same types of
complications before and after PGDT implementation.
Consider taking post-assessment measurements at 60-day, 90day, and 120-day intervals, including a minimum of 60 patients
in both the pre- and post-assessment groups.
16
Tools
• Aggregate Case Reports
• Outcome Improvement Calculator
Compare PGDT outcomes to program goals
Owner: Core Team
Review the goals defined earlier in the program and compare
results of PGDT implementation.
Tools
• Project Charter Template
Communicate results to key stakeholders
Owner: Clinical Champion
Present results to key stakeholders including the program
executive sponsor and impacted users to help sustain support
for the new PGDT clinical pathway.
Identify specific opportunities for improvement or
expansion
Consider expanding PGDT to other procedure types and/or
incorporating PGDT and the ESR Program into hospital quality
plan.
17
Tools
• PGDT Poster Template
Owner: Clinical Champion
All information provided by Edwards Lifesciences is gathered from third party sources and is presented for informational
purposes only. This information is not intended to describe, recommend, or suggest any use, feature, or benefit of any Edwards
product and does not constitute reimbursement, medical or legal advice. Edwards makes no representation or warranty
regarding this information or its completeness, accuracy or timeliness. It is not intended to make a recommendation regarding
clinical practice. Laws, regulations, and payer policies concerning reimbursement are complex and change frequently; service
providers are responsible for all decisions relating to clinical services, coding and reimbursement submissions. Accordingly,
Edwards strongly recommends consultation with payers, reimbursement specialists and/or legal counsel regarding coding,
coverage, and reimbursement matters.
Edwards, Edwards Lifesciences, and the stylized E logo are trademarks of Edwards Lifesciences Corporation.
© 2014 Edwards Lifesciences Corporation.
All rights reserved. AR10628
18