chem 210 [chapter 5: mastering nomenclature
advertisement
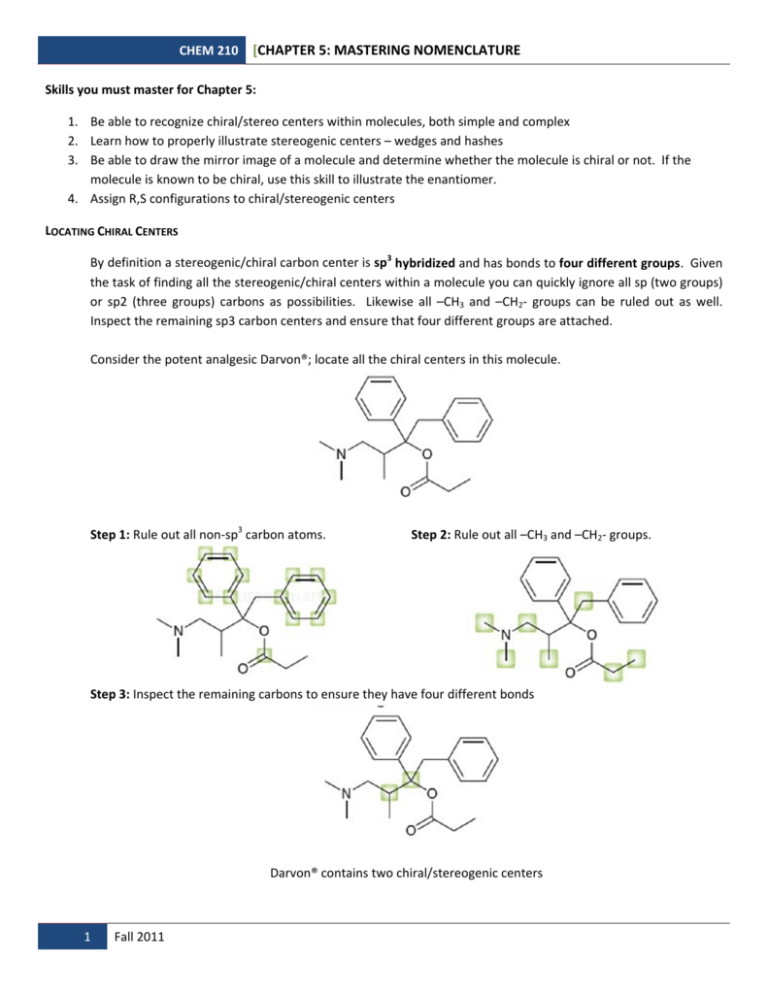
CHEM 210 [CHAPTER 5: MASTERING NOMENCLATURE Skills you must master for Chapter 5: 1. Be able to recognize chiral/stereo centers within molecules, both simple and complex 2. Learn how to properly illustrate stereogenic centers – wedges and hashes 3. Be able to draw the mirror image of a molecule and determine whether the molecule is chiral or not. If the molecule is known to be chiral, use this skill to illustrate the enantiomer. 4. Assign R,S configurations to chiral/stereogenic centers LOCATING CHIRAL CENTERS By definition a stereogenic/chiral carbon center is sp3 hybridized and has bonds to four different groups. Given the task of finding all the stereogenic/chiral centers within a molecule you can quickly ignore all sp (two groups) or sp2 (three groups) carbons as possibilities. Likewise all –CH3 and –CH2‐ groups can be ruled out as well. Inspect the remaining sp3 carbon centers and ensure that four different groups are attached. Consider the potent analgesic Darvon®; locate all the chiral centers in this molecule. Step 1: Rule out all non‐sp3 carbon atoms. Step 2: Rule out all –CH3 and –CH2‐ groups. Step 3: Inspect the remaining carbons to ensure they have four different bonds Darvon® contains two chiral/stereogenic centers 1 Fall 2011 CHEM 210 [CHAPTER 5: MASTERING NOMENCLATURE Problem 1: Find all the chiral/stereogenic centers in the following molecules 2 Fall 2011 CHEM 210 [CHAPTER 5: MASTERING NOMENCLATURE ILLUSTRATING MIRROR IMAGES The most common way we use in lecture to draw mirror images is to place a mirror to the immediate right of the structure. Keep in mind we can place the mirror anywhere to make the reflection easier. Consider the stimulant amphetamine which exists as a pair of enantiomers. To draw the enantiomer we can place the mirror in several locations: Mirror behind the molecule: Here the majority of the molecule remains the same; we only need to reverse the wedge to a dashed bond. Mirror to the side of the molecule: In this case the molecule is switched left to right and the wedge remains a wedge. Mirror beneath the molecule: Similar to the previous example except the molecule is flipped up and down. The wedge remains a wedge. As was pointed out in lecture all of these molecules are enantiomers for the amphetamine molecule pictured above: 3 Fall 2011 CHEM 210 [CHAPTER 5: MASTERING NOMENCLATURE For molecules with one chiral/stereogenic center there are some useful operations to learn regarding illustrations: Consider the following enantiomer of 2‐butanol. Remember that in skeletal structures the hydrogen is not shown on the chiral/stereogenic center: OH HO H Flip any one pair of groups about the chiral/stereogenic center you convert the molecule to its enantiomer: flip flip H OH HO H (S) (R) HO HO H (S) (R) H and so on enantiomers enantiomers Flip any two pairs of groups about the chiral/stereogenic center you simply re‐illustrate the same molecule: flip flip H OH HO H (R) (R) HO H flip HO (R) H (R) and so on flip identical identical Problem 2: Draw the enantiomer of each of the following compounds: 4 Fall 2011 CHEM 210 [CHAPTER 5: MASTERING NOMENCLATURE ASSIGNING CONFIGURATION USING THE CAHN‐INGOLD‐PRELOG PRIORITY RULES STEP 1 STEP 2 STEP 3 STEP 4 If two atoms have the Rotate or redraw the Assign priority to Identify the four each atom based on same atomic number, molecule so that atoms directly move to the next priority 4 is pointing attached to the chiral atomic number. atom out looking for backwards. Highest atomic center. number is priority 1 Be careful! Use a the first point of and the lowest method that works difference. atomic number for you! Remember multiple (usually H) receives bonds are treated as priority 4. multiple single bonds. Easy example: Assign the configuration to the chiral center in this stereoisomer of 2‐butanol: 1 4 1 3 5 Fall 2011 4 2 STEP 5 Determine whether the 1‐2‐3 sequence of priorities follows a clockwise (R) or counter‐clockwise (S) order. First find the chiral center! Before applying any of the steps, remember that the fourth bond to the chiral carbon is to a hydrogen not shown on the skeletal structure. Draw in this hydrogen using the proper wedge/dash/line. Step 1: Identify the four atoms attached to the chiral center. In this case they are C, C, O and H Step 2: Assign priority based on atomic number. O has the highest atomic number and given priority 1. Likewise H has the lowest atomic number and given priority 4. We have a tie between the two C atoms. Step 3: Moving to the next atom out from the two C atoms that are tied we see the left carbon has only hydrogens attached (lowest priority) while the right C has another C attached. We assign priority 2 to the right C and 3 to the left C. Step 4: So we have the priorities about the chiral center. In this easy example the 4 priority is already going to the back. Step 5: From 1‐2‐3 we are going clockwise and can assign this chiral center the R configuration. CHEM 210 [CHAPTER 5: MASTERING NOMENCLATURE What if the 4 priority is not going backward as drawn? What if the chiral center is difficult to visualize within a large complex molecule? There are two methods to choose from. Use the method that is easiest for you or apply the best method to the situation. Method 1: Most common problem‐The 4 priority is pointing forward. Solution: Do not redraw the molecule. Instead determine whether 1‐2‐3 is clockwise or counter‐clockwise as drawn and reverse the assignment. OH Draw in missing H - should be wedge OH H Assigning priorities: 3 1 OH H 4 2 - remember C=C becomes C C C Going from 1-2-3 we are going clockwise 1 OH H 4 3 2 Normally clockwise is R, but since priority 4 (H) is pointing towards us we reverse the answer. The configuration of this center is S. Method 2: Redraw the center as a separate structure using only the priority numbers. Remember flipping two pairs of bonds re‐illustrates the same configuration. Do this to get the 4‐priority pointing backwards or to make the configuration easier to visualize. Step 1: Step 2: Step 3: 6 Fall 2011 CHEM 210 [CHAPTER 5: MASTERING NOMENCLATURE Problem 3: Assign configurations to the chiral centers in the following molecules: Challenge Problem: If you get this one you have it mastered! Remember that you have a 50:50 chance of guessing right even if your analysis is incorrect, so don’t run off and party yet…. 7 Fall 2011 CHEM 210 [CHAPTER 5: MASTERING NOMENCLATURE Last skill: Drawing molecules of a particular configuration. This may be the hardest to give you a definitive set of rules for solving. If you look at the molecules you’ve been assigned so far you see that chirality usually involves a functional group and more specifically a heteroatom (non‐C atom). In most of these structures it is customary to use wedges or dashes for the heteroatoms (usually priority 1) or hydrogens (usually priority 4) and leave the carbons in the plane of the paper wherever possible. The easiest way for novices to start is to simply draw one enantiomer and assign the configuration. If the configuration does not match the one you were instructed to draw, simply flip one pair of bonds to invert the center. Problem 4: Draw molecules of the indicated configuration for the chiral center: b. a. as both R and S (label each) as both R and S (label each) c. d. Naproxen – Draw S Aspartic acid – Draw S f. CH3CHFBr – Draw R e. Carvone – Draw R 8 Fall 2011 CHEM 210 [CHAPTER 5: MASTERING NOMENCLATURE KEY Problem 1: Problem 2: 9 Fall 2011 CHEM 210 [CHAPTER 5: MASTERING NOMENCLATURE Problem 3: Challenge Problem: Problem 4: 10 Fall 2011