Dr. King's Chem 122 Additional Practice 1. Draw a structural formula
advertisement

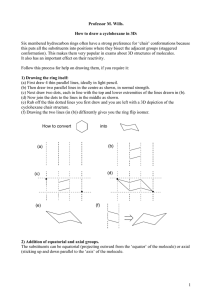
Dr. King’s Chem 122 Additional Practice 1. Draw a structural formula for: a) 4-ethyl-2,4-dimethylnonane b) 4-isopropyl nonane c) trans-1,3-dimethylcyclobutane d) 2,2,4,4-tetramethyloctane A C B D 2. Name the following compounds. Give the number of primary, secondary and tertiary carbons and units of unsaturation in each. cis-1,3,5-trimethylcyclohexane 6-tert-butyl- 7-cyclohexyl-4-cyclopropyl-2-methyldecane *when alphabetizing substituents hyphenated prefixes (tert, n, sec…) don’t matter 3. Draw the 2 chair conformations for cis-1-isopropyl-3-methylcyclohexane and trans-1isopropylcylohexane, indicate which is more stable for each. CIS H H H3C CH3 H H H H H H H H H H H H H H H H H H BOTH EQUATORIAL BOTH AXIAL BIGGER GRP EQUATORIAL LESS STABLE TRANS H H H H H H H3C H H CH3 H H H H H H H H H H 1 AXIAL, 1 EQUATORIAL H H 1 AXIAL, 1 EQUATORIAL BIGGER GRP AXIAL LESS STABLE 4. Draw Newman projections and sketch an approximate relative energy diagram for the conformers resulting from rotation about the C2-C3 bond in 2,3-dimethylbutane and in C2-C3 of the propane chain n-propylcyclohexane. TH ISISH O W TH EC O M P U TERD R AW SEC LIP SED ID EN TIC AL H H H H H H H H H H A A B ID EN TIC AL B D H C E H w o sst-ebdow thitm eotthhyelr's lm e c ietrp h hyls E A F b ee stth -o n l,y2pairsofgauche m y l s not3inarow THIS IS HOW THE COMPUTER DRAWS ECLIPSED IDENTICAL H H H H H H H H H H H A B C D H E F IDENTICAL worst-both methyl's eclipsed with other methyls best-only 2 pairs of gauche methyls, not 3 in a row A B C D E F A IDENTICAL H H H H H H H H H H H B A H H H best---anti B C D E H D C IDENTICAL A H H H H H F H H H E H F WORST-methyl and cyclohexane eclipsed