Organic Chemistry I: Cycloalkane Stability and Conformations October 31, 2008
advertisement
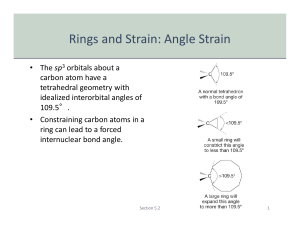
Organic Chemistry I: Cycloalkane Stability and Conformations October 31, 2008 Dr. Milkevitch Chapter 3 1 Cycloalkane Stability • 5- and 6-membered rings • Most commonly found • Most stable • But Why? • Adolf Von Baeyer-1905 • Attempted to explain relative stabilities of cyclic alkanes • Reasoned that non-cyclic (acyclic) alkanes had bond angles of 109.5° • Bond angles of 109.5° puts electrons in bonds as far apart as possible » Most stable Chapter 3 2 Cycloalkane Stability •However, if a cyclic alkane has bond angles other than 109.5 °: •Orbitals of carbon-carbon bonds cannot: •Achieve optimum overlap •There must be some angle strain •Example: Cyclobutane •90° bond angles Chapter 3 3 Cycloalkane Stability •Also notice: •Newman projection: bonds are eclipsed •Gives rise to torsional strain •It all adds up: •Angle strain + torsional strain = Ring strain. Chapter 3 4 How Ring Strain is Measured •Heats of combustion •Amount of heat released •When a compound is burned in an excess of O2 •Produce a table of molar heats of combustion •Divide heat of combustion by the number of CH2 groups •Energy per CH2 groups Chapter 3 5 Heats of Combustion/CH2 Alkane + O2 CO2 + H2O 697.1 686.1 664.0 658.6 kJ Ring Strain per CH2 Long-chain 38.5 27.5 5.4 663.6 kJ/mol 662.4 658.6 0 3.8 5.0 => Chapter 3 6 Focus on Cyclohexane • Combustion data shows no ring strain present • Must have bond angles near 109.5° (no angle strain) • Must have no eclipsing of bonds (no torsional strain) • What if cyclohexane were planar? • Makes sense, doesn’t it? • Bond angles would be 120°, but should be 109.5° • implies some angle strain • Planar ring would have eclipsed bonds on CH2 groups • Implies some torsional strain • Conclusion: • Cyclohexane ring cannot be planar • Cyclohexane must have some type of alternate confirmation(s) • There are two major conformations of cyclohexane => Chapter 3 7 Chair Conformer Bond angles = 109.5° Dr. Milkevitch in his office Staggered conformation => Chapter 3 8 Boat Conformer Bond angles = 109.5° Adopts “twist” boat, to relieve torsional => strain Chapter 3 9 Cyclohexane Conformations •At any instant: •Most molecules in chair confirmation •Energy barrier between chair & boat sufficiently low •Conformations interconvert several times per second •Interconversion: •“Footrest” of chair flips up, planar with sides of molecule •Forms “half-chair” conformation •High energy process Chapter 3 10 Conformational Energy => Chapter 3 11 Axial and Equatorial Positions •Examine cyclohexane in chair confirmation •2 different kinds of hydrogen •First kind: •6 bonds (one on each carbon) •Directed up and down •Parallel to axis of ring •Called axial hydrogens •Second Kind: •6 other bonds (one on each carbon) •Point out from ring •Called Equatorial hydrogens Chapter 3 12 Axial and Equatorial Positions => Chapter 3 13 Monosubstituted Cyclohexanes => Chapter 3 14 Monosubstituted Cyclohexanes •A substituent on a cyclohexane ring (chair conformation) •Can occupy: •An axial position •An equatorial position •However, at RT there are 2 chair conformations •In equilibrium •Called a “chair-chair” interconversion •“Chair-chair” interconversion: •Changes axial to equatorial, and vice-versa Chapter 3 15 Monosubstituted Cyclohexanes •But which is the lowest energy? •Substituent in equatorial position Chapter 3 16 Substituent in Axial Position •2 gauche interactions Chapter 3 17 Substituent in Equatorial Position •No gauche interactions Chapter 3 18 1,3-Diaxial Interactions •Gauche interactions of axial substituent •Places electron clouds near to each other => •Form of steric hinderance Chapter 3 19 Disubstituted Cyclohexanes •Very severe interaction when large groups are axial •More stable interaction: both groups equatorial => Chapter 3 20 Disubstituted Cyclohexanes •What happens when both groups cannot be equatorial? •In other words, one group axial, one group equatorial •Interconversion, get the same thing Chapter 3 21 Disubstituted Cyclohexanes •Result: More stable conformation •Larger group equatorial •Smaller group axial Chapter 3 22 Recognizing Cis-Trans Isomers •Each ring carbon has 2 available bonds •One up, one down H Up CH3 CH3 CH3 down = Up down CH3 H Trans-1,2 dimethylcyclohexane Chapter 3 23 Bulky Groups • Groups like t-butyl cause a large energy difference between the axial and equatorial conformer. • Most stable conformer puts t-butyl equatorial regardless of other substituents. => Chapter 3 24 Bicyclic Alkanes • Fused rings share two adjacent carbons. • Bridged rings share two nonadjacent C’s. bicyclo[3.1.0]hexane Chapter 3 bicyclo[2.2.1]heptane => 25 Naming Bicyclic Alkanes •Based on the name of the alkane •Having the same number of carbons in the ring system •Name follows prefix bicyclo •Set of brackets with 3 numbers •Corresponds to the number of carbons in the bridges Chapter 3 26 Naming Bicyclic Alkanes •All fused and bridged cyclic systems have 3 bridges connecting the 2 bridgehead atoms •Numbers in brackets give the number of carbon atoms •In each of the 3 bridges connecting the bridgehead carbons •In order of decreasing size Bridgehead carbons Chapter 3 27 End of Chapter 3 Chapter 3 28