two branches of science
advertisement

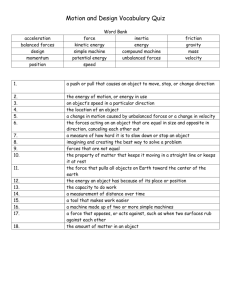
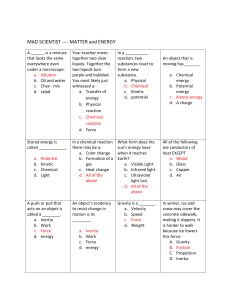
Integrated Science Midterm Exam Review Sheet Science Department • The midterm will cover the following Chapters: Chapter 1 Chapter 2 Chapter 3 Chapter 4 Chapter 5 Chapter 8 Chapter 9 Introduction to Science Matter and Energy Atoms and the Periodic Table Structure of Matter Balancing Chemical Equations Motion Work and Energy • The Midterm is a combination of five parts. There will be 50 multiple choice including calculations, 16 matching, 20 true/false, 14 on reading a diagram, and 5 essays. • You will need to bring a pencil to the exam for the scantron section and a pen for the rest of the exam. You will also need to have with you a calculator for the calculation section. • You will be given a periodic table and also a ion chart. • Talking will not be allowed at any time during the exam. HOW TO PREPARE • You should reread all the chapters and review your class notes. • Study your chapter tests and study guides. Many similar questions will come from those materials. • Study the chapter review at the end of the chapters and key terms. Key Terms and Practice Questions/Problems Chapter 1 Scientific Law Scientific Theory Two Branches of Science Three Branches of Natural Science Scientific Method Metric System Scientific Notation Significant figures SI base units Review Understanding Concepts Page 27 Density problems page 62 #16-21 Chapter 2 Matter Chemical and Physical Changes States of Matter Law of conservation of mass Compounds and Elements Homogenous and Heterogeneous Mixtures Phase changes Review Understanding Concepts Page 61 Chapter 3 Dalton’s Theory Rutherford Proton Valence electron Atomic number Democritus Thomson Neutron Ion Mass Number Bohr’s Model Atoms Electron Isotopes Periodic Table and Families Review Understanding Concepts Page 101 Math Skills Page 100 # 6-9 Chapter 4 Chemical bonds Metallic bonds Naming Mole Ionic Bonds Molecule Formula Writing Molar Mass Review Understanding Concepts Page 101 Check your understanding page 128 #1-4 Mole Calculations Covalent Bonds Polyatomic ions Chapter 5 Chemical Reactions Endothermic Reactions Balancing Exothermic Reactions All Reaction Types Review Understanding Concepts Page 177 Balancing Equation Work Sheets Chapter 8 Speed Momentum Force Unbalanced forces Air-resistance Weight Terminal velocity Velocity Acceleration Balanced forces Friction Gravity Newton’s 3 laws of motion Review Understanding Concepts Page 275 Velocity, Acceleration, Weight, Force, Momentum problems 15-26 page 276 Chapter 9 Work Simple Machines Plane Family Power Levers Potential Energy Mechanical Advantage Pulley Kinetic Energy Review Understanding Concepts Page 315 Work, Power, Kinetic and Potential Energy Review page 315 #16-18 • • • • • • • • • • • Essay Section Topics Precision vs accuracy Chemical vs physical changes. Compare/Contrast and give examples Free fall acceleration in reference to falling objects of different weights. Terminal Velocity and motion Organization of the Periodic table. Major families. Comparing metals and nonmetals . The steps of scientific method Pure Science vs Applied Science Ionic vs Covalent bonds in terms of their properties Work vs Power Chemical Reactions and signs that take place Isotopes and mass. Atoms versus ions. Good luck with your studies. Science Department MIXED PROBLEMS How many Significant Figures? 1. 256.09 ___________ 2. 0.00000026 ___________ 3. 0.00470 ___________ 4. 2400 ___________ Place the following numbers in scientific notation: 23400000000 ______________ 0.00000056 ______________ Classify the what following units indicate. 1. 2. 3. 4. 5. 6. 7. 8. mi/hr min N J m kg W kg • m/s ______________ ______________ ______________ ______________ ______________ ______________ ______________ _____________ Identify the equations. 1. density ______________ 2. power ______________ 3. acceleration ______________ 4. velocity ______________ 5. Kinetic energy _____________ 6. Potential Energy ___________ 7. work ____________ 8. force/weight ____________ 9. momentum ___________ 1. The mass of a solid piece of metal is 11 g and has a volume of 4.1 cm3. What is the density of the piece of metal? 2. A chemist needs 25.0 g of bromine for an experiment. What volume should she use if the density of bromine is 3.12 g/cm3? 3. Calculate the momentum of a 9.1 kg toddler who is riding in a car moving east at 89 km/hr. 4. A block pushed with a force of 13.5 N accelerates at 6.5 m/s2 to the left. What is the mass of the block? 5. Simpson drives his car with an average velocity of 85 km/hr toward the east. How long will it take him to drive 560 km on a perfectly straight highway. 6. You an two friends apply a force of 425 N to push a piano up a 2.0 m long ramp. If you make it to the top in 5.0 s, what is your power output in watts? 7. A 2.0 kg rock sits on the edge of a cliff 12 m above the beach. A) Calculate the potential energy of the system. B) What will its kinetic energy be just before it hits the beach? C) Calculate the speed of the rock just before it hits the beach. (HINT: see practice hint page 301)