Kinetic vs. Thermodynamic Control: Lab Report
advertisement

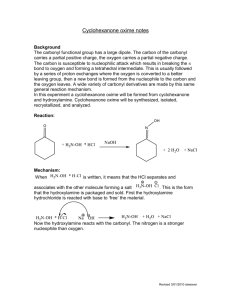
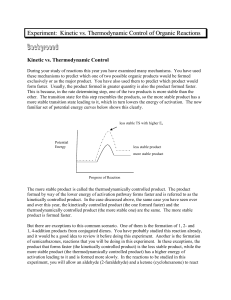
Kinetic versus Thermodynamic Control in Competing Reactions Introduction: The purpose of this experiment is to test kinetic theory by running several reactions and determine which product are formed under kinetic conditions and which products are formed under thermodynamic conditions. Kinetically controlled products are those which have the lowest transition states between competitive reactions; this would be represented by TS1 in the above diagram. At low temperatures, this makes it easier for the reaction to overcome the activation barrier ensuring that the kinetic product is formed faster. Thermodynamically controlled products are those which have a more stable product (P2) due to the product energy level being lowest between competitive reactions. Therefore at high temperatures, the thermodynamic product is dominant because it is possible to reach the higher transition state. It is in essence easier to reach the activation energy barrier. In Part A of the experiment, two reactions are carried out to synthesize semicarbazones to be used in Part C of the experiment. The first reaction being the preparation of cyclohexanone semicarbazone; The second being the preparation of furfural semicarbazone; These reactions will be further analyzed under thermo conditions and kinetic conditions in part B of this experiment. To test this theory experimentally, in Part B of the experiment, two competing reactions are carried out; Cyclohexanone, furfural and semicarbazide are reacted, initially without heat and then again this reaction is carried out with heat. The significance of this process is to determine which product is formed under kinetic conditions (forms faster at low temperatures) and which product is formed under thermodynamic conditions (forms slower but is the major product at high temperatures). Experimental: The purpose of Part A of the experiment is to furfural and cyclohexanone semicarbazones for part C of the experiment. 50 mmol of semicarbazide hydrochloride along with 10.0g of sodium bicarbonate are mixed in 125 ml of deionized water in an Erlenmeyer flask. Once effervescence ceases, 50 mmol of cyclohexanone is added and swirled in the flask. Once a precipitate forms, the mixture is then vacuum filtered and the product isolated. The product is then recrystallized from deionized water. The melting and yield is then recorded This procedure is then repeated for preparation of the furfural semicarbazone (50 mmol of furfuraldehyde is used instead of 50 mmol of cyclohexanone). Part B of the experiment is to truly demonstrate the fundamental theory behind competitive reactions and thermo vs kinetic control. 10 mmol of semicarbazide hydrochloride is mixed with 2.0g of sodium bicarbonate and 25 ml of deionized water in a 125 ml Erlenmeyer flask. Once effervescence has ceased, 10 mmol cyclohexanone and 10 mmol of furfuraldehyde are added to the solution. Once all reactants are properly mixed and a precipitate forms, the mixture is then vacuum filtered to isolate a product. This product is the recrystallized from deionized water. The product is then verified by measuring the melting point and mixed melting point. This procedure is then repeated under heating for about 1 hour. Part C of the experiment is to verify kinetic vs thermo control of part B through interconversion reactions. 10 mmol of cyclohexanone semicarbazone (obtained from Part A) is mixed with 10 mmol of furfuraldehyde in 20 ml of deionized water in a 125 ml Erlenmeyer flask. This mixture is placed in a heat bath for roughly 1 hour. The mixture is then cooled to room temperature and the precipitate is then vacuum filtered and recrystallized from deionized water. The product is then identified using melting point and mixed melting point. This procedure is then repeated using 10 mmol of furfuraldehyde semicarbazone also obtain from Part A and cyclohexanone instead of 10 mmol of cyclohexanone semicarbazone and furfuraldehyde. Results: Competitive Reaction Yield A-1 Theoretical:7.76g Actual: 6.047g Yield A-2 Theoretical: 7.66g Actual : 5.254g Discussion: The purpose of this experiment was to test the theory of kinetic vs thermo control by carrying out reactions under specific conditions to yield specific products. Part A of the experiment consisted exclusively of preparing the semicarbazones to be used for the experiment. In A-1, 5.794 g of semicarbazide hydrochloride was mixed with 9.990 g of sodium bicarbonate in 125 ml of deionized water. It is important to note that the semicarbazide is chlorinated because the amino group would otherwise be easily protonated, so it is easier to handle when chlorinated. Upon addition of water, an intense amount of effervescence was observed. Once effervescence has ceased, 5.185 ml of cyclohexanone was added to the solution. A foamy solution followed after the addition of cyclohexanone and the mixture was then vacuum filtered. Once isolated, the product is then recrystallized using deionized water to remove further impurities and a melting point range of (168-170) °C was recorded. The literature value for the melting point of cyclohexanone semicarbazone is 166°C which would indicate that the product obtained is in fact cyclohexanone semicarbazone. The reason for which the melting point of the actual product obtained is slightly higher is due to trace amounts of impurities that were not filtered out or still remained in the reaction flask during and after recrystallization. Part A-2 followed in a similar procedure to A-1 however, instead of using cyclohexanone, 4.147 ml of furfuraldehyde was added along with 10.0 g of sodium bicarbonate, 5.765 g of semicarbazide hydrochloride and 125 ml of deionized water. This mixture however did not show effervescence as in the first part and the solution displayed a yellow colour. Once filtered and recrystallized, a melting point of 199.2°C was recorded. The literature value for the melting point of furfural semicarbazone is 202 which would indicate that the product obtained is in fact furfural semicarbazone. It is speculated that the reason for the melting point being slightly lower than literature value is due to trace amounts of impurities in the final product. Part B of the experiment exclusively deals with competitive reactions where the formations of different semicarbazones are attained through different reaction conditions. Altering reaction conditions can give rise to specific products predominating given the specific reaction condition. The first part of B was performed under conditions with no heat. 1.115 g of semicarbazide hydrochloride was mixed with 2.0g of sodium bicarbonate in 25 ml of water. Not much effervescence was observed and the solution remained clear. 1.036 ml of Cyclohexanone was then added along with 0.829 ml of furfuraldehyde. With the addition of these two reagents, the competitive reaction then begins. One of two products can predominate; cyclohexanone semicarbazone or furfural semicarbazone. Since the reaction condition is at low temperature, whichever product forms the fastest and is the major product will in fact be the kinetic product. Once the reaction was complete and the product was isolated through vacuum filtration and recrystallized from deionized water. A melting point range of (156-158) °C was recorded which is closest to the literature value of cyclohexanone semicarbazone (166°C) which indicates that this semicarbazone is the product. The second part of B is performed in much the same way however, the reaction conditions are different and heat is introduced. 1.117 g of semicarbazide was mixed with 1.998g of sodium bicarbonate in 25 ml of deionized water. Again there was little effervescence, then 1.036 ml of cyclohexanone and 0.829 ml of furfuraldehyde were added and the reaction commenced under heating for approximately 1 hour. Note that heating for this period of time is to ensure that a specific product predominates. Since the variable of heat was introduced, reaching the higher transition state is easier to accomplish (as in TS2) and the thermodynamic product should predominate; Once the reaction had completed and the product had been isolated through vacuum filtration and recrystallized from deionized water, a melting point range of (196-198°C) was recorded. This value is approximately the literature value of furfural semicarbazone (202°C) which indicates that this particular semicarbazone is the most stable product, is favoured at high temperatures, and is the thermodynamic product. Part C of the experiment is done to further prove the results obtained in Part B of the experiment through interconversion reactions. In C-1, 1.025 g of cyclohexanone semicarbazone (kinetic product) was used along with 0.829 ml of furfuraldehyde in 20 ml of deionized water. This mixture was then heated for roughly 1 hour, isolated through vacuum filtration and then recrystallized from deionized water. A melting point range of (186-189) °C which is close the literature value of furfural semicarbazone implies that the kinetic product interconverts to the thermo product (the most stable) under heating and in the presence of furfuraldehyde. The lower melting point range obtained is due to impurities present in the product. In C-2, 1.254 g of the thermo product was used along with 1.036 ml of cyclohexanone and 20 ml of deionized water. As in C-1, the mixture was then heated for roughly 1 hour, isolated through vacuum filtration and recrystallized from deionized water. A melting point range of ( ) was observed which implies that again, the thermo product (furfural semicarbazone) predominates under these conditions involving higher temperatures. Conclusion: In conclusion, cyclohexanone forms faster under kinetic conditions (lower temperatures) due to a lower transition state than that of furfural semicarbazone. Furfural semicarbazone is the most stable product and is favoured at high temperatures, it is the thermodynamic product. Things For Your Consideration (1) The transition state of product B in figure (i) is higher than that of Product A, therefore product B cannot be kinetically controlled. Product B is also overall less stable than that of A due to a higher final product energy level, therefore it neither thermodynamically controlled. (2) In part B-1, cyclohexanone semicarbazone was formed faster therefore it is kinetically controlled. In part B-2, furfural semicarbazone was the major product therefore it is thermodynamically controlled. In both parts of C, the thermo product predominates (due to heating), which is furfural semicarbazone. (3) (a) An increase in the amount of semicarbazide will not alter the data for either product (cyclohexanone or furfuraldehyde would be limiting). (b) Doubling the volumes of the aldehyde or ketone will not affect the yield (semicarbazide would be limiting)