Chemistry Worksheet: Atomic Structure, Changes, Trends
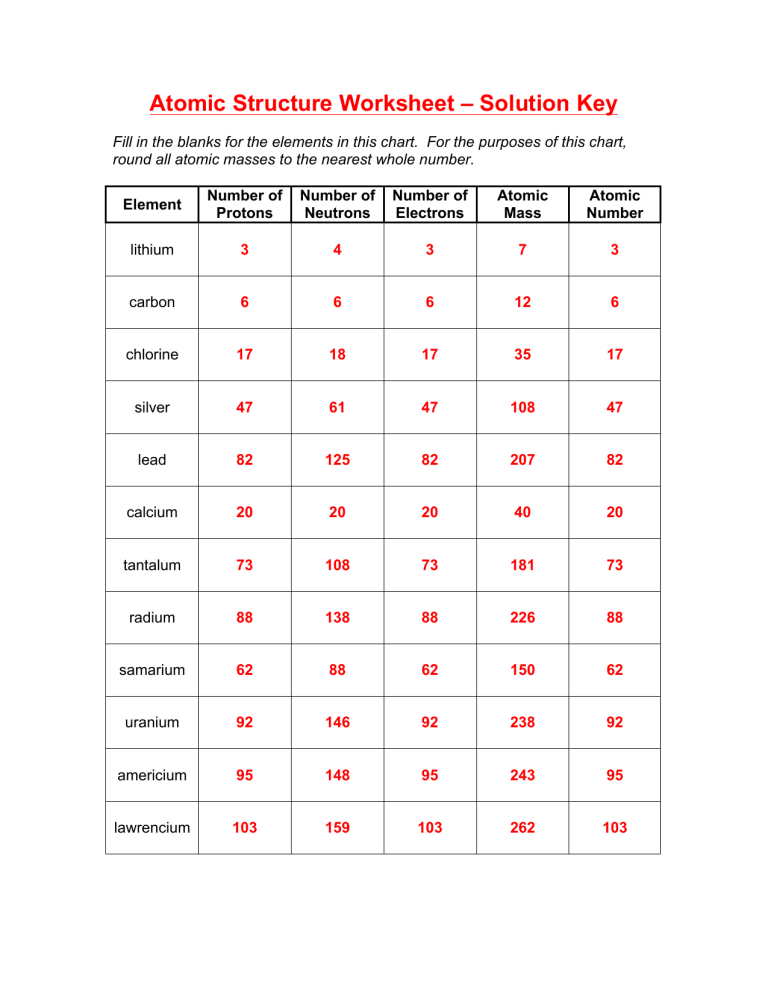
Atomic Structure Worksheet – Solution Key
Fill in the blanks for the elements in this chart. For the purposes of this chart, round all atomic masses to the nearest whole number.
Element lithium carbon radium samarium uranium americium lawrencium chlorine silver lead calcium tantalum
Number of
Protons
3
6
17
47
82
20
73
88
62
92
95
103
Number of
Neutrons
4
6
18
61
125
20
108
138
88
146
148
159
Number of
Electrons
3
6
17
47
82
20
73
88
62
92
95
103
Atomic
Mass
7
12
35
108
207
40
181
226
150
238
243
262
Atomic
Number
3
6
17
47
82
20
73
88
62
92
95
103
Chemical and Physical Change Homework
For each of the following, determine whether a chemical or physical change is taking place:
1) A hot dog is cooked
• Chemical or physical change? Answer may vary, depending on whether the student is used to boiling or grilling a hot dog.
However, cooking is generally thought of as being a chemical change.
•
Your reason: The properties of the hot dog (consistency, taste, etc) are different after cooking than before, suggesting that drastic changes have been made.
2) Thousand Island dressing and mayonnaise are mixed to make “secret sauce” for hamburgers
• Chemical or physical change? Physical change
•
Your reason: Mixing things doesn’t break or make chemical bonds
3) Water is boiled in preparation for making pasta
•
Chemical or physical change? Physical change
•
Your reason: The process of boiling doesn’t break the bonds in water molecules (breaking the hydrogen bonds between molecules doesn’t count)
4) Old ham goes bad in the refrigerator
•
Chemical or physical change? Chemical change.
•
Your reason: From the wide variety of properties that change, it can be inferred that the chemical structure of the ham has been changed.
5) A rock star gets a tattoo on his forehead
• Chemical or physical change? Physical change
Your reason: The dye in tattooing ink doesn’t formally bond to the skin. Students may not be aware of this, so if they say that it’s a chemical change but have the general idea that the ink has bonded, they should still receive credit.
Periodic Trends Worksheet - Solutions
1) Rank the following elements by increasing atomic radius: carbon, aluminum, oxygen, potassium.
From smallest to largest: oxygen < carbon < aluminum < potassium
2) Rank the following elements by increasing electronegativity: sulfur, oxygen, neon, aluminum.
From smallest to largest: neon < aluminum < sulfur < oxygen
3) What is the difference between electron affinity and ionization energy?
Electron affinity is a measure of the energy change that occurs when an atom grabs an electron. Ionization energy is a measure of how much energy it takes to pull electrons off of an element. Both values are for atoms in the gas phase.
4) Why does fluorine have a higher ionization energy than iodine?
It is harder to pull electrons off of fluorine because fluorine has a higher electronegativity than iodine. Iodine has a much lower electronegativity than fluorine because of the shielding effect, which states that the electrons in inner energy levels tend to push electrons in outer energy levels away from the nucleus. This pushing makes it harder for iodine to grab electrons.
5) Why do elements in the same family generally have similar properties?
Because they have similar electron configurations and the same number of valence electrons. Because valence electrons are responsible for most of the chemistry we observe, this similarity causes the properties of the elements to also be similar.
For chemistry help, visit www.chemfiesta.com © 2002 Cavalcade Publishing – All Rights Reserved








