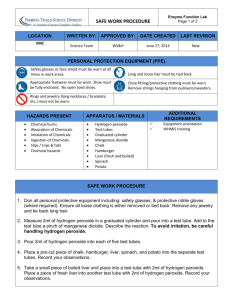
Enzyme Catalyst Lab (Teacher Copy)

Enzyme Catalyst Lab (Teacher Copy)
Florida Sunshine State Standards Benchmarks:
SC.A.1.4.4 The student experiments and determines that the rates of reaction among atoms and molecules depend on the concentration, pressure, and temperature of the reactants and the presence or absence of catalysts. AA
SC.A.1.4.2 The student knows that the vast diversity of the properties of materials is primarily due to variations in the forces that hold molecules together. CS
Objective:
SC.A.1.4.4 I can determine the effect of concentration on rate of reaction.
Background Knowledge:
In order to stay alive, organisms need to carry out reactions that require energy. Some chemical reactions that make life possible are too slow or have activation energies that are too high to make them practical for living tissue. Enzymes are proteins that speed up chemical reactions by lowering the activation energy of the reaction.
The enzyme catalase speeds up the breakdown of hydrogen peroxide (H
2
O
2
) into water
(H
2
O) and oxygen gas (O
2
). The reaction is described by the following equation:
1
Engage:
To peak student interest, they will observe what happens when hydrogen peroxide is added to potato and liver in separate beakers.
Problem Statement:
How will the different concentrations of hydrogen peroxide affect the rate of the reaction in the experiment?
Hypothesis:
If I increase the concentration of hydrogen peroxide (independent variable), then the rate of reaction (dependent variable) will increase.
Materials:
•
Yeast solution (dry yeast will be dissolved and suspended in water with some sucrose one hour before the lab)
•
Eight 50 mL beakers (as needed)
•
Five test tubes with stoppers
•
Test tube rack
•
3% hydrogen peroxide
•
Distilled water
Explore:
Procedures:
•
•
•
•
•
Filter paper disks (coffee filters may be used) – cut slightly smaller than the diameter of the test tube
Marking pencil
Forceps
Paper towels
Optional (For demo: potato, liver and
2 beakers)
1.
Prepare peroxide solutions in separate test tubes as outlined in Table 1 below:
Concentration Hydrogen Peroxide Distilled Water
0% Peroxide Solution
25% Peroxide Solution
50% Peroxide Solution
75% Peroxide Solution
100% Peroxide Solution
0 mL
9 mL
18 mL
27 mL
35 mL
35 mL
27 mL
18 mL
9 mL
0 mL
2
2. Using the forceps, dip a filter paper disk into the beaker containing the solution of activated yeast. Keep the disk in the solution for 4 seconds, and then remove it.
3. Place the disk on a paper towel for 4 seconds to remove any excess liquid.
4. Using the forceps, transfer the filter paper disk to the bottom of a rubber stopper.
Note: The yeast solution contains the catalase enzyme and when the enzyme soaked disk comes into contact with hydrogen peroxide, the reaction results in the formation of oxygen bubbles.
5. Insert the stopper into one of the test tubes and quickly invert the test tube.
Have one person in your group measure how long it takes for the bubbles to carry the disk to the top of the test tube. Record the time in a data table similar to the one shown below.
6. Repeat steps 2-5 until you have a minimum of three trials for each peroxide solution.
7. Calculate the average rising time for each of the peroxide solutions. Record this information in your data table.
8. Construct a graph plotting the concentration of hydrogen peroxide on the x-axis
(independent variable) and rising time on the y-axis (dependent variable).
Explain:
Collect Data: Table 2
Rising Time (seconds)
Beaker Trial 1 Trial 2 Trial 3 Average
0% Hydrogen Peroxide
25% Hydrogen Peroxide
50% Hydrogen Peroxide
75% Hydrogen Peroxide
100% Hydrogen Peroxide
3
Analyze Data
• Construct and analyze the graph from table 2.
Students should plot an x-y axes graph using results from Trials 1-3 and interpret their results.
Conclusion
1. Suppose you had placed a filter paper disk in a 30% hydrogen peroxide solution.
Using your graph, predict how long it would take this disk to rise to the top.
Answers will vary. Have students make assumptions based on their results in Table 2, but it should center around the reaction occurring faster because the concentration of the reactant (hydrogen peroxide) increased.
2. In a paragraph, describe how the concentration of hydrogen peroxide affects the breakdown rate of hydrogen peroxide. Use the results of this experiment to justify your answer.
Answers will vary. The higher the concentration of catalase, the faster the decomposition of hydrogen peroxide. Students should use supporting details from their results to support their answer.
3. In a paragraph explain why your hypothesis was or was not supported.
My hypothesis can be supported because as the concentration of hydrogen peroxide increased, the faster the reaction occurred. Students should use results to support their hypothesis from the experiment.
Closure Activity
• Discuss each group’s results with the class as a whole. Make sure to discuss and correct any misconceptions the students have.
• Write down the WHOLE class’s conclusions on the board and refer back to the hypotheses.
4
Elaborate:
Extension/Teacher Notes
• Time permitting, the students may choose to repeat the experiment with even higher or lower concentrations.
• Before passing out the labs, have the students think about what processes in the body use enzymes to function. Be sure to discuss as a class the vital enzymes for human digestion such as protease, amylase, lipase, etc.
• Discuss other factors that affect the rate of reactions.
• Teachers should utilize the Limiting Reactants Gizmo from www.explorelearning.com
Evaluate:
Catalase is found in plant and animal cells and protects them from a dangerous waste chemical by breaking it down into water and oxygen. What is the waste chemical?
A.
Hydrogen Peroxide
B.
Nitrogen Dioxide
C.
Lactic Acid
D.
Carbon Monoxide
5
Scientist: ____________________________ Date: ____________ Period: _____
Enzyme Catalyst Lab (Student Copy)
Objective:
SC.A.1.4.4 I can determine the effect of concentration on rate of reaction.
Background Knowledge:
In order to stay alive, organisms need to carry out reactions that require energy. Some chemical reactions that make life possible are too slow or have activation energies that are too high to make them practical for living tissue. Enzymes are proteins that speed up chemical reactions by lowering the activation energy of the reaction.
The enzyme catalase speeds up the breakdown of hydrogen peroxide (H
2
O
2
) into water
(H
2
O) and oxygen gas (O
2
). The reaction is described by the following equation:
Problem Statement:
6
Hypothesis:
If (independent variable), then (dependent variable)
Materials:
•
Yeast solution (dry yeast will be dissolved and suspended in water with some sucrose one hour before the lab)
•
Eight 50 mL beakers (as needed)
•
Five test tubes with stoppers
•
Test tube rack
•
3% hydrogen peroxide
•
Distilled water
•
Filter paper disks (coffee filters may be used) – cut slightly smaller than the diameter of the test tube
•
Marking pencil
•
Forceps
•
Paper towels
•
Optional (For demo: potato, liver and
2 beakers)
7
Procedure:
1.
Prepare peroxide solutions in separate test tubes as outlined in Table 1:
Table 1
Concentration Hydrogen
Peroxide
Distilled Water
0% Peroxide Solution
25% Peroxide Solution
0 mL
9 mL
35 mL
27 mL
50% Peroxide Solution
75% Peroxide Solution
18 mL
27 mL
18 mL
9 mL
100% Peroxide Solution 35 mL 0 mL
2. Using the forceps, dip a filter paper disk into the beaker containing the solution of activated yeast. Keep the disk in the solution for 4 seconds, and then remove it.
3. Place the disk on a paper towel for 4 seconds to remove any excess liquid.
4. Using the forceps, transfer the filter paper disk to the bottom of a rubber stopper.
Note: The yeast solution contains the catalase enzyme and when the enzyme soaked disk comes into contact with hydrogen peroxide, the reaction results in the formation of oxygen bubbles.
5. Insert the stopper into one of the test tubes and quickly invert the test tube.
Have one person in your group measure how long it takes for the bubbles to carry the disk to the top of the test tube. Record the time in a data table similar to the one shown below.
6. Repeat steps 2-5 until you have a minimum of three trials for each peroxide solution.
7. Calculate the average rising time for each of the peroxide solutions. Record this information in your data table.
8. Construct a graph plotting the concentration of hydrogen peroxide on the x-axis
(independent variable) and rising time on the y-axis (dependent variable).
8
Data:
Beaker
0% Peroxide
Table 2
Rising Time (seconds)
Trial 1 Trial 2 Trial 3
25% Peroxide
50% Peroxide
75% Peroxide
100% Peroxide
Construct and analyze the graph from your Table 2
Average
Graph Analysis:
9
Results and Conclusions:
1. Suppose you had placed a filter paper disk in a 30% hydrogen peroxide solution.
Using your graph, predict how long it would take this disk to rise to the top.
2. In a paragraph, describe how the concentration of hydrogen peroxide affects the breakdown rate of hydrogen peroxide. Use the results of this experiment to justify your answer.
3. In a paragraph, explain why your hypothesis was or was not supported.
10
4.
Discuss each group’s results with the class as a whole. Make sure to discuss and correct any misconceptions the students have.
5.
Write down the WHOLE class’s conclusions on the board and refer back to your hypothesis.
6.
Catalase is found in plant and animal cells and protects them from a dangerous waste chemical by breaking it down into water and oxygen. What is the waste chemical?
A.
Hydrogen Peroxide
B.
Nitrogen Dioxide
C.
Lactic Acid
D.
Carbon Monoxide
11