Evaporation & Intermolecular Forces Lab Experiment
advertisement

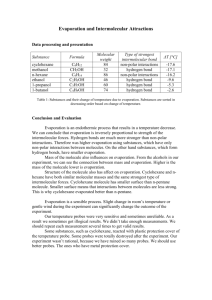
Name:____________________________________ Class Period:________ Date:_____________ Chemistry Laboratory Experiment Investigating Evaporation and Intermolecular Forces Introduction Evaporation is an endothermic process. Energy is required to overcome the intermolecular forces (IMF) between the liquid molecules so they can escape into the vapor state. The stronger the intermolecular forces, the more energy is required. The energy for evaporation comes from the surroundings, causing a cooling effect. This is how perspiring helps you cool – water on your forehead evaporates cooling your forehead in the process. Animals cool off in a variety of ways (pigs roll in mud, dogs pant, etc.) but each method depends in part upon the endothermic process of water evaporation. The rate of evaporation is inversely proportional to the strength of the intermolecular forces. In other words, the rate of evaporating will be faster with a substance that has weak intermolecular forces. A fast rate of evaporation will result in a larger temperature drop than a slow rate of evaporation for equal volumes of liquid. Have you ever had your arm wiped with rubbing alcohol before a shot? It feels cooler than water evaporation because the alcohol has weaker intermolecular forces and evaporates faster than water. Recall that the strongest type of intermolecular force is hydrogen bonding, which occurs between molecules that have a hydrogen atom bonded to a small electronegative atom like oxygen, nitrogen or fluorine. Dipoledipole forces occur between the positive end of a polar molecule and the negative end of another polar molecule. All molecules posses London dispersion forces, which are the result of the electron cloud of a molecule becoming instantaneously polarized and inducing a polarized electron cloud of a neighboring molecule. The more massive the molecule the larger the electron cloud and the more polarizable it is, leading to greater London dispersion forces. This is the only type of intermolecular force between nonpolar molecules. In general, polar molecules have stronger intermolecular forces than nonpolar molecules. In this laboratory experiment you will predict and measure the cooling effect of different liquids. Your knowledge of Lewis structures, VSEPR theory, molecular geometry and intermolecular forces will allow you to rate the polarity of the liquids. The type of IMF, along with molar mass, will allow you to predict the relative cooling effect of the different Adapted from Flinn Experiment 9: Evaporation and Intermolecular Attractions; Chemistry with Computers, 2 nd Edition; 2000. ICLCS Team 4: The 3-Ds Jeanne M. Capriotti ; Bradley-Bourbonnais Community High School; 2008 ©2011 University of Illinois Board of Trustees • http://islcs.ncsa.illinois.edu/copyright Name:____________________________________ Class Period:________ Date:_____________ liquids. Then you will test your prediction by measuring a temperature drop during evaporation. Pre-lab For each of the liquids in the table below, draw the structure. Use WebMO to confirm the Lewis structure that you drew. Determine the molar mass and indicate whether the molecule is polar or nonpolar and state the type of intermolecular forces that are present. Liquid Formula Methanol CH3OH Ethanol CH3CH2OH n-propanol CH3CH2CH2OH n-butanol CH3CH2 CH2CH2OH n-pentane CH3CH2 CH2CH2CH3 Molar Mass g/mol Lewis Structure Polar or Predominate Nonpolar Type of IMF Adapted from Flinn Experiment 9: Evaporation and Intermolecular Attractions; Chemistry with Computers, 2 nd Edition; 2000. ICLCS Team 4: The 3-Ds Jeanne M. Capriotti ; Bradley-Bourbonnais Community High School; 2008 ©2011 University of Illinois Board of Trustees • http://islcs.ncsa.illinois.edu/copyright Name:____________________________________ Class Period:________ Date:_____________ Liquid Formula Hexanes CH3 (CH2 )4CH3 Heptanes CH3 (CH2 )5CH3 Octane CH3(CH2)6 CH3 Glycerine OHCH2CH(OH)CH2OH Water HOH Acetone CH3COCH3 Molar Mass g/mol Lewis Structure Polar or Type of IMF Nonpolar Adapted from Flinn Experiment 9: Evaporation and Intermolecular Attractions; Chemistry with Computers, 2 nd Edition; 2000. ICLCS Team 4: The 3-Ds Jeanne M. Capriotti ; Bradley-Bourbonnais Community High School; 2008 ©2011 University of Illinois Board of Trustees • http://islcs.ncsa.illinois.edu/copyright Name:____________________________________ Class Period:________ Date:_____________ Underneath the liquid name, indicate the order of rate of evaporation that you expect (1 = fastest and 11 = slowest) and the dipole moment (D) from WebMO. Materials Filter paper cut into 2.5 x 2.5 cm pieces Vernier temperature probe, interface, Logger Pro and computer or Digital thermometer and stopwatch or glass thermometer and stopwatch Small rubber bands (tubing cut in thin sections) Masking tape Test tubes with stoppers Test tube holder Part A - Effect of Molar Mass - Liquids: methanol, ethanol, n-propanol, n-butanol Part B – Effect of Molar Mass – Liquids: pentane, hexanes, heptanes, octane Part C – Effect of Number of Hydrogen Bonds – Liquids: glycerin , water, methanol, hexane Part D – Effect of Type of Intermolecular Force – Liquids: water, n-propanol, acetone, hexanes Safety Caution! The liquids used in this experiment are flammable. There should be no open flames or sources of sparks near the liquids. Avoid inhaling the vapors or contact with your skin or clothing. Let your teacher know immediately if a spill or accident occurs. Obtain and wear safety glasses and lab aprons. Procedure Your teacher will assign the parts of this experiment that you will be doing. If you will not be doing all parts, then the data obtained from each group will be shared. The procedure for each liquid of each part is identical and is given below. With care, two liquids can be done at once. 1. Obtain stoppered test tubes containing about 3 mL each of the liquids that you will measure. Make sure that they are marked so that you can identify them. 2. Obtain as many pieces of 2.5 cm x 2.5 cm filter paper as liquids that you will test. Adapted from Flinn Experiment 9: Evaporation and Intermolecular Attractions; Chemistry with Computers, 2 nd Edition; 2000. ICLCS Team 4: The 3-Ds Jeanne M. Capriotti ; Bradley-Bourbonnais Community High School; 2008 ©2011 University of Illinois Board of Trustees • http://islcs.ncsa.illinois.edu/copyright Name:____________________________________ Class Period:________ Date:_____________ 3. Obtain or cut 2 small rubber bands and 2 pieces of masking tape. 4. Secure the filter paper to the probe or thermometer. Make sure to only cover the top portion of the filter paper so as to allow for evaporation. 5. Place the wrapped probe or thermometer in the test tube with the liquid. After one minute record the initial temperature as the “time zero” temperature in the data table. 6. Quickly remove the probe or thermometer and place it on the lab bench so that the filter paper end hangs over the edge. Secure in place with the masking tape. 7. Record the temperature every 10 seconds until the temperature reaches a minimum and then begins to increase again or for 5 minutes, whichever comes first. Make sure to avoid breezes and do not blow on the filter paper to make it evaporate faster. 8. Repeat this process with the next liquid. 9. Clean up according to your teachers instructions. 10. Obtain and share data with your classmates as directed by your teacher. Calculation Table Record the molar mass and temperature change for each liquid. Liquid Temperature change = tinitial-tminimum (Co) Molar Mass (g/mol) Methanol (A, C) Ethanol (A) Propanol (A, D) Butanol (A) Pentane (B) Hexane (B, C, D) Heptanes (B) Octane (B) Glycerine (C) Water (C, D) Acetone (D) Adapted from Flinn Experiment 9: Evaporation and Intermolecular Attractions; Chemistry with Computers, 2 nd Edition; 2000. ICLCS Team 4: The 3-Ds Jeanne M. Capriotti ; Bradley-Bourbonnais Community High School; 2008 ©2011 University of Illinois Board of Trustees • http://islcs.ncsa.illinois.edu/copyright Name:____________________________________ Class Period:________ Date:_____________ Analysis of Data 1. Make a graph of temperature change vs. molar mass for the “A” solvents. Connect the data points with a smooth curve. To the graph add the “B” solvents. Make sure to include a key with your graph. (This is done most easily using Excel.) 2. Make a graph of the change in temperature vs. the number of hydrogen bonds for the “C” solvents. Questions 1. From the graph for the “A” and “B” liquids, how did molar mass affect the rate of evaporation? Remember a small temperature difference indicates a slow rate of evaporation. 2. From the graph of liquids “C” what can you conclude about the number of hydrogen bonds and the rate of evaporation? 3. What is the main difference between liquids “A” and liquids “B”? 4. For liquids “D” was the most polar solvent the one with the slowest evaporation rate? Was the least polar compound the one with the fastest evaporation rate? Explain your answer. Include discussion of the dipole moments obtained from WebMO and the type of intermolecular forces. 5. Based on your data, what do you think has a greater effect – molar mass or type of intermolecular force? Explain and justify your answer. Adapted from Flinn Experiment 9: Evaporation and Intermolecular Attractions; Chemistry with Computers, 2 nd Edition; 2000. ICLCS Team 4: The 3-Ds Jeanne M. Capriotti ; Bradley-Bourbonnais Community High School; 2008 ©2011 University of Illinois Board of Trustees • http://islcs.ncsa.illinois.edu/copyright Name:____________________________________ Class Period:________ Date:_____________ 6. Did the order of evaporation rate that you indicated in the pre-lab exercise, correspond with your results. Please indicate any differences and offer an explanation for the discrepancy. 7. What are some sources of error in this lab? Data Table “A” Liquid Methanol Ethanol Propanol Butanol Time (s) Temperature (oC) Temperature (oC) Temperature (oC) Temperature (oC) 0 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 160 Adapted from Flinn Experiment 9: Evaporation and Intermolecular Attractions; Chemistry with Computers, 2 nd Edition; 2000. ICLCS Team 4: The 3-Ds Jeanne M. Capriotti ; Bradley-Bourbonnais Community High School; 2008 ©2011 University of Illinois Board of Trustees • http://islcs.ncsa.illinois.edu/copyright Name:____________________________________ Class Period:________ Date:_____________ 170 180 190 200 210 220 230 240 250 260 270 280 290 300 Data Table “B” Liquid Pentane Hexane Heptane Octane Time (s) Temperature (oC) Temperature (oC) Temperature (oC) Temperature (oC) 0 10 20 30 40 50 60 70 80 90 100 110 120 130 140 Adapted from Flinn Experiment 9: Evaporation and Intermolecular Attractions; Chemistry with Computers, 2 nd Edition; 2000. ICLCS Team 4: The 3-Ds Jeanne M. Capriotti ; Bradley-Bourbonnais Community High School; 2008 ©2011 University of Illinois Board of Trustees • http://islcs.ncsa.illinois.edu/copyright Name:____________________________________ Class Period:________ Date:_____________ 150 160 170 180 190 200 210 220 230 240 250 260 270 280 290 300 Data Table “C” Liquid Glycerine Water Propanol Hexane Time (s) Temperature (oC) Temperature (oC) Temperature (oC) Temperature (oC) 0 10 20 30 40 50 60 70 80 90 100 110 120 Adapted from Flinn Experiment 9: Evaporation and Intermolecular Attractions; Chemistry with Computers, 2 nd Edition; 2000. ICLCS Team 4: The 3-Ds Jeanne M. Capriotti ; Bradley-Bourbonnais Community High School; 2008 ©2011 University of Illinois Board of Trustees • http://islcs.ncsa.illinois.edu/copyright Name:____________________________________ Class Period:________ Date:_____________ 130 140 150 160 170 180 190 200 210 220 230 240 250 260 270 280 290 300 Data Table “D” Liquid Water Acetone Propanol Hexane Time (s) Temperature (oC) Temperature (oC) Temperature (oC) Temperature (oC) 0 10 20 30 40 50 60 70 80 90 100 110 Adapted from Flinn Experiment 9: Evaporation and Intermolecular Attractions; Chemistry with Computers, 2 nd Edition; 2000. ICLCS Team 4: The 3-Ds Jeanne M. Capriotti ; Bradley-Bourbonnais Community High School; 2008 ©2011 University of Illinois Board of Trustees • http://islcs.ncsa.illinois.edu/copyright Name:____________________________________ Class Period:________ Date:_____________ 120 130 140 150 160 170 180 190 200 210 220 230 240 250 260 270 280 290 300 Adapted from Flinn Experiment 9: Evaporation and Intermolecular Attractions; Chemistry with Computers, 2 nd Edition; 2000. ICLCS Team 4: The 3-Ds Jeanne M. Capriotti ; Bradley-Bourbonnais Community High School; 2008 ©2011 University of Illinois Board of Trustees • http://islcs.ncsa.illinois.edu/copyright