Evaporation and Inte..
advertisement
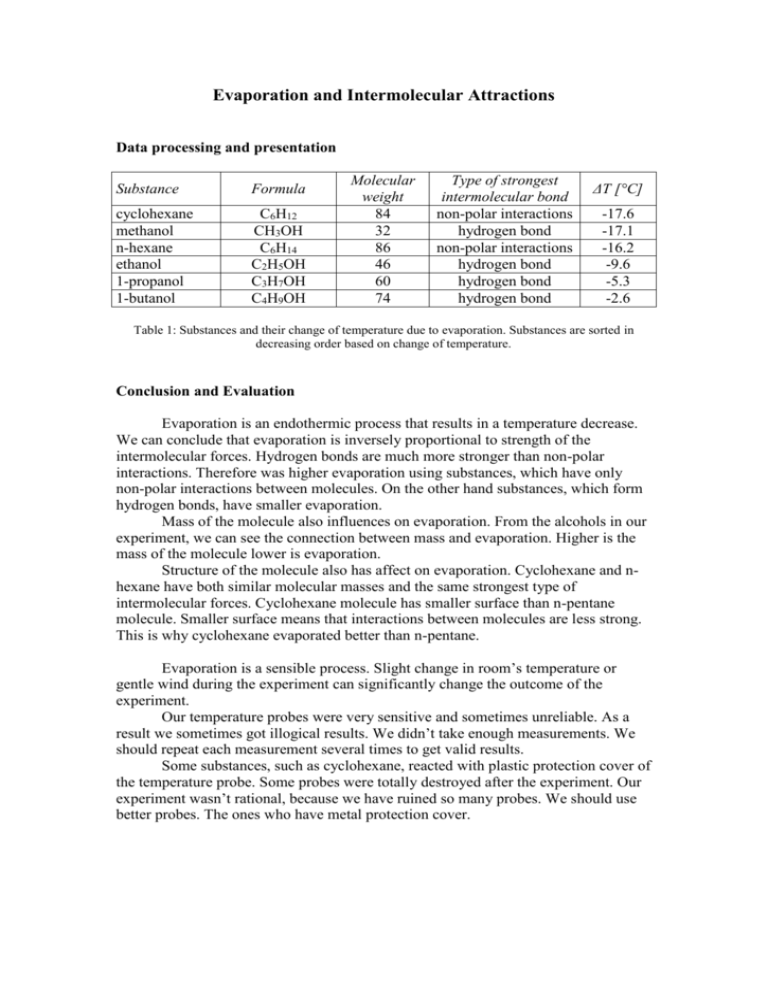
Evaporation and Intermolecular Attractions Data processing and presentation Substance Formula cyclohexane methanol n-hexane ethanol 1-propanol 1-butanol C6H12 CH3OH C6H14 C2H5OH C3H7OH C4H9OH Molecular weight 84 32 86 46 60 74 Type of strongest intermolecular bond non-polar interactions hydrogen bond non-polar interactions hydrogen bond hydrogen bond hydrogen bond ΔT [°C] -17.6 -17.1 -16.2 -9.6 -5.3 -2.6 Table 1: Substances and their change of temperature due to evaporation. Substances are sorted in decreasing order based on change of temperature. Conclusion and Evaluation Evaporation is an endothermic process that results in a temperature decrease. We can conclude that evaporation is inversely proportional to strength of the intermolecular forces. Hydrogen bonds are much more stronger than non-polar interactions. Therefore was higher evaporation using substances, which have only non-polar interactions between molecules. On the other hand substances, which form hydrogen bonds, have smaller evaporation. Mass of the molecule also influences on evaporation. From the alcohols in our experiment, we can see the connection between mass and evaporation. Higher is the mass of the molecule lower is evaporation. Structure of the molecule also has affect on evaporation. Cyclohexane and nhexane have both similar molecular masses and the same strongest type of intermolecular forces. Cyclohexane molecule has smaller surface than n-pentane molecule. Smaller surface means that interactions between molecules are less strong. This is why cyclohexane evaporated better than n-pentane. Evaporation is a sensible process. Slight change in room’s temperature or gentle wind during the experiment can significantly change the outcome of the experiment. Our temperature probes were very sensitive and sometimes unreliable. As a result we sometimes got illogical results. We didn’t take enough measurements. We should repeat each measurement several times to get valid results. Some substances, such as cyclohexane, reacted with plastic protection cover of the temperature probe. Some probes were totally destroyed after the experiment. Our experiment wasn’t rational, because we have ruined so many probes. We should use better probes. The ones who have metal protection cover.