MODEL OF MUSCLE-TENDON INTERACTION DURING FROG
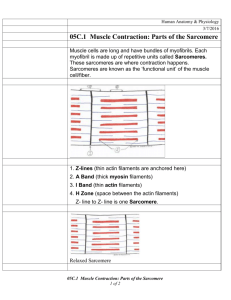
advertisement

OOZI-9290192SS.oO+.OO
‘(:” 1992 Pergamon Press plc
J. Biomechonics Vol. 25, No. 4, pp. 421428. 1992.
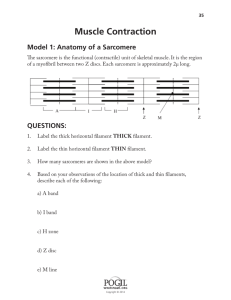
Printed in Great Britain
MODEL OF MUSCLE-TENDON
INTERACTION DURING
FROG SEMITENDINOSIS FIXED-END CONTRACTIONS
RICHARD L. LIEBER*, CYNTHIA G. BROWN and CHRISTINE L. TRESTIK
Department of Orthopaedics and Rehabilitation, Biomedical Sciences Graduate Group, Veterans
Administration Medical Center and University of California, San Diego, CA 92161, U.S.A.
Abstract-A structural model was developed to explain sarcomere shortening at the expense of tendon
lengthening in the frog semitendinosis (ST) muscle=-tendon system. The model was based on the data of
Lieber et al. [Am. J. Physiol. 261, C&C92 (1991)], who determined the relationship between the sarcomere
length, tendon load (as a fraction of maximum isometric tension) and tendon, bone-tendon junction (BTJ),
and aponeurosis strain. The model was generated assuming a finite time-course of cross-bridge attachment
[Huxley, Prog. Biophys. 7,255-318 (1957)], an ideal sarcomere length-tension relationship [Gordon et al.,
J. Physiol. 184, 170-192 (1966)] and an ideal force-velocity relationship [Katz, J. Physiol. %, 454 (1939);
Edman, J. Physiol. 291,143-159 (1979)]. Functionally, sarcomeres operated on three distinct regions of the
length-tension curve: (1) regions where the muscle force decreased as sarcomeres shortened (the shallow and
steep ascending limbs); (2) regions where the muscle force increased as sarcomeres shortened and there was
little passive tension (descending limb, where sarcomere length < 3.0 pm); and (3) regions where the muscle
force increased as sarcomeres shortened and there was a significant passive tension (descending limb where
sarcomere length > 3.0 Pm). Using such a physiological model, it was found that the effect of tendon
compliance was to ‘skew’ the sarcomere length-tension curve to the right and to increase the operating range
of the muscle-tendon unit. Thus, maximum tension in the muscle occurred at an active sarcomere length of
2.0-2.2 pm, whereas in the muscle-tendon system, the maximum tension occurred at a longer resting
sarcomere length of about 2.5 Pm. The degree to which the tendon affected the muscle system depended on
its material properties and dimensions. These data suggest that tendons are not merely rigid links connecting
muscles to bones, but impart distinct properties to the muscular system.
INTRODUCTION
models exist which describe the relationship between skeletal muscle and force production.
Such models range from formulations of cross-bridge
attachment and detachment rates (Huxley, 1957;
Squire, 1990) to phenomenological models of muscle
force output as a function of length, activity, and
velocity input (Hatze, 1973; Zajac, 1989). In all models
muscle force varies as a function of length (Gordon et
al., 1966) and velocity (Katz, 1939), which is expected
for the muscle contractile component. However, a
whole muscle is not simply an amplified sarcomere.
Muscle has significant series elasticity within and
outside it (Morgan, 1976; Rack and Westbury, 1984).
In fact, recent studies demonstrated that skeletal
muscle-tendon
units may have unique properties
compared to the properties of muscle alone (Walmsley
and Proske, 1981; Zajac, 1989; Hoffer et al., 1989).
Therefore, models which are useful in describing normal movement must account for both muscle and
tendon properties as well as their interaction. The
recent mammalian muscle-tendon
model by Zajac
(1989) is generic in that it was designed to apply to any
muscle-tendon
actuator given appropriate scaling
factors. While it admirably accomplishes its purpose,
it is not possible to simply apply that model to any
muscle-tendon unit in any species since the issues of
Numerous
Received infinalform 25 July 1991.
*Author to whom correspondence should be addressed.
scaling and species specificity come into play
(Schmidt-Nielsen, 1984). Since we were interested in
the behavior of the frog semitendinosis (ST) during
normal locomotion (Mai and Lieber, 1990), our purpose was to develop a model for this particular
muscle-tendon actuator that was based on experimental data in order to determine the tendon’s influence in this system. A brief report of this work has
appeared elsewhere (Lieber and Leonard, 1989).
METHODS
Biomechanical experiments
The model chosen for this study was the dorsal head
of the frog semitendinosis (ST) muscle-tendon unit
(Rana pipiens). This model was chosen based on the
muscle’s well-established sarcomere length-tension
properties (Gordon et a/., 1966) and previous studies
establishing the relationship between muscle and joint
properties (Lieber and Boakes, 1988; Mai and Lieber,
1990).
Frogs were sacrificed by double pithing (n= 14
independent experiments) and the ST-tendon unit was
carefully isolated along with its attachments ko the
pelvis and tibia. The bones of the bone-muscletendon (BMT) unit were clamped to specially designed
fixtures which permitted viewing of the bone-tendon
interface while maintaining secure contact with the
BMT unit (Lieber et al., 1991). The BMT unit was
submerged in chilled Ringer’s solution adjusted to
pH = 7.0. One clamp was fixed to the moving arm of a
421
422
R. L. LIEBERet al.
A
B
Contractile Component (CC)
Parallel Elastic Component (PEC)
Fig. 1. (A) Schematic diagram of the frog semitendinosis muscle-tendon unit drawn to scale. Values shown
at the top of the figure are lengths in mm (mean + SD. for 14 specimens) of the muscle fiber, aponeurosis,
tendon, and bone-tendon junction (BTJ). Note that the ratio of muscle fiber to connective tissue is 1.5
(calculated based on relative lengths as { [2.8 x 2]+ [2.1 x 23 + 5.5}/10.5), rendering this system relatively
‘stiff’ as defined by Zajac (1989). (B) Mechanical analog representing theoretical model. Muscle contractile
component with ideal length-tension and force-velocity properties is represented by a schematic sarcomere.
servo motor which permitted simultaneous control of force and measurement of displacement (Cambridge Technology Model 310, Watertown, MA). Dye lines (elastin stain) were applied at
intervals along the BMT unit partitioning it into three
regions: a region containing the bone-tendon interface
(referred to as the bone-tendon junction), a region
containing only the bare tendon (tendon), and a region
containing the muscle-tendon junction (aponeurosis).
Boundaries between these regions were defined somewhat arbitrarily based on morphological appearance.
Muscle length was set to L,, the length at which
twitch tension was maximal. This occurred at a nominal sarcomere length of 2.45 pm, approximately in the
midpoint of sarcomere lengths achievable in the frog
semitendinosis with various hip and knee joint configurations (see Fig. 4A of Mai and Lieber, 1990). Passive
tension at this length was near the noise level of the
transducer (about 100 pg). Following the measurement of maximum tetanic tension (P,), muscles were
passively loaded to P, and the strain (a) was measured
in three different regions of the connective tissue
(Fig. 1A): the muscle-tendon junction (aponeurosis),
the tendon, and the bone-tendon junction (BTJ). The
average load-strain function for each connective tissue region was calculated (Fig. 2) and it was determined that there was no significant difference between
the tendon and bone-tendon junction regions.
dual-mode
0
I
2
3
1
6
6
6
Fig. 2. Average load-strain relationship for the three different connective-tissue regions studied. In this experiment, the
aponeurosis was significantly more compliant than either the
tendon or bone-tendon junction (P<O.O5), which were not
significantly different from one another. At maximum tetanic
tension, the average strain in the aponeurosis, bone-tendon
junction, and tendon were 8, 3.4, and 2%, respectively.
series arrangement of muscle fibers with two lengths of
tendon and one length of aponeurosis (Fig. IB). The
equations describing the passive properties of each of
these components were:
Tendon:
p,(%p,)=
10(~+0.633)/1.35,
Aponeurosis:
~~(%~~)=10(~+3.63)/5.66,
Model assumptions
In developing the model for fixed-end muscle contraction, each muscle-tendon unit was modelled as a
7
Strain (%)
(I)
(2)
Muscle:
~,,,(~~p~)=
IO~(sL-~~~O)/l.O7~-2.14,
(3)
Model of frog muscle-tendon
where E represents the strain, %P, represents the
relative maximum tetanic tension, and SL represents
the sarcomere length (in pm). Since the passive properties of the connective tissue were known [Table 1,
equations (l)-(3)], the remainder of the model was
generated based on the following assumptions:
(1) Muscle fibers have an ideal length-tension relationship as described by Gorden et al. (1966).
(2) Muscle fibers have an ideal force-velocity relationship for shortening as described by Katz (1939),
Edman (1979) and Morgan et al. (1982), where the
force-velocity constants are: a = 0.25 and b = 0.25.
(3) Connective tissue in series with muscle fibers has
load-strain properties according to Lieber et al. (1991)
[equations (1) and (2)].
(4) Muscles have a sarcomere-length-passivetension relationship according to Lieber et al. (1991)
[equation (3)].
(5) The time course of muscle activation follows the
time course of cross-bridge attachment as indicated by
Huxley’s parameterf, for frog skeletal muscle at 12°C
(Huxley, 1957). For the strains observed during fixedend contractions, the parameter g would be negligible.
The cross-bridge attachment rate was scaled so that
the muscle would be maximally activated after 100 ms
according to the equation:
Activation fraction =
No muscle series elastic component (SEC) was
incorporated into the model. This was because there is
some uncertainty as to the physical location of frog
muscle SEC (e.g. compare Julian and Morgan, 1981
and Ford et al., 1980). In addition, since the displacement required to drop the muscle fiber force to zero is
only 3-4 nm/half sarcomere (Ford et al., 1977), we felt
that muscle SEC would be dominated by tendon and
aponeurosis compliance. Of course, this model structure will thus tend to underestimate
sarcomere
shortening slightly. We thus bias the results toward
showing no effect of tendon compliance rather than
overemphasizing it. However, tendon and aponeurosis strain will not be affected by this assumption.
Using the above assumptions, a FORTRAN program was developed (Fig. 3) which simulated a fixedend tetanic contraction. The logical program flow
proceeded as follows: following initialization of the
experimental parameters such as connective tissue
material
properties,
sarcomere
length
and
muscle-tendon dimensional quantities (Fig. 3, box l),
resting tendon, aponeurosis and muscle fiber lengths
were calculated
using experimentally
obtained
load-strain values (Fig. 3, box 2). The muscle was then
activated according to the data of Huxley (1957) (Fig.
3, box 3). As the muscle developed tension appropriate
to the specified sarcomere length, a certain amount of
passive tension resisted sarcomere shortening according to assumption (4) (Fig. 3, boxes 46). This tension
strained both the tendon and aponeurosis according
interactions
423
Table 1. Muscle-tendon properties used in our model*
Parameter
Value
Muscle properties
Muscle length (mm)
Fiber length (mm)
Sarcomere number
Maximum tetanic tension (N)
Sarcomere length at L, (pm)
TendonJAponeurosislBTJ
22.5_+1.7
10.5+ 1.4
4781+647
0.366+0.17
2.45 + 0.06
properties
Tendon length (mm)
BTJ length (mm)
Aponeurosis length (mm)
Tendon Young’s modulus at P, (MPa)
2.11 +0.63
2.80 +0.52
5.51 f 1.10
188+?1
*Values shown represent the mean k standard deviation
for the same 14 specimens for which stress-strain data were
obtained. Physiological CSA is the muscle physiological
cross-sectional area as calculated using the equations of
Sacks and Roy (1982). BTJ refers to the bone-tendon junction region as described in Methods section. P, is the
maximum tetanic tension, Lo is the muscle length at which
the tension is maximum. Data from Lieber et al. (1991).
to their relative stiffnesses and was distributed accordingly (Fig. 3, boxes 4,5). This tension was compared to
the isometric tension for that sarcomere length and
level of activation to determine the relative isometric
tension (Fig. 3, box 7). Using the force-velocity relationship (Katz, 1939), sarcomere shortening velocity
was then calculated (Fig. 3, box 8) and the new
sarcomere length calculated (based on the sarcomere
shortening velocity and time interval of 0.01 ms). This
process was repeated in 0.01 ms increments (no significant difference in the results was obtained for time
intervals ranging from 0.00-1.0 ms, data not shown)
and sarcomeres continued to shorten until muscle
contractile force was equivalent to the resistive force of
the connective tissue. At this point, the velocity was
equal to zero (Fig. 3, box 10). Throughout the activation scheme, sarcomere length and velocity, muscle
active tension, muscle passive tension, and tendon
tension were recorded to permit complete description
of sarcomere shortening at the expense of tendon
lengthening during fixed-end contractions.
Sarcomere shortening at the expense of tendon
lengthening was calculated for sarcomere lengths
ranging from 1.3-3.7 pm for the ST and, for comparative purposes, was also calculated for muscle-tendon
systems with different lengths of tendon and aponeurosis.
RESULTS
As expected, sarcomere shortening and magnitude
were dependent on initial sarcomere length. At very
long sarcomere lengths, relatively little shortening
occurred while at shorter sarcomere lengths, shortening often exceeded 0.2 pm (Fig. 4A). At very long
sarcomere lengths where passive tension was appreciable (e.g. sarcomere lengths greater than 3.1 pm), as
424
R. L. LIEBERet al.
I
Initialize Parameters
Q
I
.I
1 I
Calculate Length ok
Tendon
Aponeurosis
I
Musde
l
l
21
l
Begin Activation
I
l
l
l
Tendon
Aponeurosis
Muscie
f-l1
5
Calculate Total Tension
6
Calculate Relative
Isometric Tension
7
4
Calculate Isotonic
ShorteningVelocity
8
1
Calculate New
SarcomereLength
I Yes
Fig. 3. Flow chart of muscle-tendon
contraction algorithm. See text for calculation details.
Model of frog muscle-tendon
interactions
425
1207
i?
.**.
100.
.
8.
80.
l
.
.
.
.
s
'B
5
60.
/\
tp
.
.
.
40.
a
6
20.
0
0
100
200
10
300
15
20
++*
\:. ,
.+.***
2.5
30
35
40
Sarcomere Length (pm)
Time (ms)
Fig. 6. Sarcomere length-tension relationship for an ideal
muscle fiber (solid line) and muscle fiber in series with a
tendon of the properties measured (filled circles). Note that
the sarcomere length-tension relationship is skewed to the
right because sarcomeres which begin at lengths above the
optimum (i.e. slightly longer than 2.0-2.2 pm) are allowed to
shorten to higher regions of the descending limb or even onto
the plateau of the sarcomere length-tension curve. Passive
tension (P,,,) is shown in crosses.
Sarcomere Length (pm)
cause shortening
Fig. 4. (A) Sarcomere length vs time for sarcomere lengths
ranging from 1.3-3.7 pm in 0.2 pm increments. Ending sarcomere length is shown to the right of the trace. (B) Identical
data as (A) but plotting sarcomere length vs tension. Note
that sarcomeres shorten until the velocity equals zero, at
which point they have ‘touched’ a point on the
length-tension curve.
Initial Sarcomere Length = 3.0 pm
0
loo
203
XPI
Time (ms)
Fig. 5. Sample time course of muscle fiber, aponeurosis and
tendon length change for an initial sarcomere length of
3.0pm. Note that the aponeurosis deforms more than the
tendon since it is longer and more compliant. Deformation
magnitude is (AL) shown for each region to the right of the
trace.
sarcomeres shortened on the descending limb of the
length-tension
relationship, they became stronger due
to the increasing filament overlap and decreasing
velocity (Fig. 4B). It is interesting to note that, as
sarcomeres shortened in these regions of high passive
tension, the muscle contractile component (CC, Fig.
1B) was required to develop relatively large tensions to
shortening
since some force was lost by the
of the muscle parallel elastic component
(PEC, Fig. 1B). Thus, as the CC developed tension, it
had to exceed the force lost by the PEC in order for
shortening to occur! At shorter sarcomere lengths
where, as sarcomeres shortened, they became potentially weaker (e.g. sarcomere lengths less than 2.0 pm),
tension increased with shortening, primarily due to
increasing activation (Fig. 4B).
Using this algorithm, it was possible to plot the time
course of length change in muscle, tendon, and aponeurosis for any sarcomere length (Fig. 5). Note that
for a typical fixed-end contraction (e.g. at a sarcomere
length of 3.0 pm) tendon strain was less than aponeurosis strain due to its higher stiffness. The relative
lengthening of the two connective tissue components
resulted directly from their values of relative stiffnesses
which changed with load.
By varying the initial sarcomere length, we constructed a sarcomere length-tension curve where sarcomere length represented that at the beginning of the
contraction for a system with series compliance (Fig.
6). Note that the ‘ideal’ sarcomere length-tension
curve (i.e. the one described by Gordon et al., 1966)
(Fig. 6, solid line) was distorted to larger sarcomere
lengths as a result of series compliance (Fig. 6, circles).
Thus, in the muscle-tendon system, maximum tetanic
tension (P,) would occur between sarcomere lengths
of 2.3 and 2.4 pm (Fig. 6). In fact, this was not
significantly different from the resting sarcomere
length at P, of 2.45kO.06 pm measured by Lieber et
al. (1991). At both long and short sarcomere lengths,
the amount of shortening was relatively small-although for different reasons: at short sarcomere
lengths, with almost no passive resistance, sarcomeres
could shorten only a small amount before they entered
the ‘delta state’ (Ramsey and Street, 1940). At large
426
R. L. LIEBERet al.
sarcomere lengths, with high passive tension, sarcomere shortening was relatively small because sarcomeres could generate only a small amount of active
tension (Pee), and because any shortening decreased
muscle fiber tension due to decreased P,nc. In other
words, shortening tended to decrease P,nc faster than
sarcomere
it increased P,,. Finally, at intermediate
lengths, as sarcomeres shortened, they first increased
the potential tension with shortening (on the descen-
ding limb of the length-tension curve) and then decreased the potential tension with shortening (on the
steep and
shallow
ascending
limb
of the
length-tension
curve), but in all cases shortening
occurred until total muscle tension (Pc, + P,,,) was
equal to the resisting force of the connective tissue (PA
or PT). Thus, the relationship
between the initial
sarcomere length and sarcomere length change grossly
resembled an inverted parabola (Fig. 7).
In addition to the sarcomere length-tension
curve,
it was also possible to calculate a muscle-tendon
length-tension curve (Fig. 8). By adding series com-
I?
j
%
5
5
f.
P
3
9
El
8
z
cn
o.3
.
O2
.
.***.
l.
.
.
.
.
Q’-
.
.
.
.
.
g
.
.
I
1.5
*
2.0
2.5
2.0]A
.
.
007
1.0
pliance, the operating range of the muscle-tendon unit
actually increased by about 1 mm! In other words, by
having a compliant connection with the bone, the
muscle-tendon unit increased its operating range to a
length greater than that which would be expected
based simply on the number of sarcomeres in the
muscle fibers!
As mentioned, the frog ST muscle-tendon actuator
is considered stiff due to the short connective tissue
length/fiber length ratio (1.5; Fig. 1). In order to
simulate other muscle-tendon units, the ratio was
varied from 1 to 15 and the resulting change in the
sarcomere length (Fig. 9A) as well as the sarcomere
length-tension relationship (Fig. 9B) were constructed. Note that, as connective tissue length increased,
the magnitude of sarcomere shortening permitted also
increased (Fig. 9A). Note also that the sarcomere
length which shortened the greatest amount shifted to
longer lengths as the connective tissue length increased. This is because longer sarcomeres were allowed to shorten to optimal sarcomere length
(2.2 pm). Similarly, as the connective tissue length
increased, the length-tension relationship skewed further to the right and narrowed as more and longer
sarcomeres were allowed to shorten to or past optimal
sarcomere length (Fig. 9B).
8
3.0
.
.
- I. ’
3.5 4.0
Initial Sarcomere Length (pm)
Fig. 7. Sarcomere length change during fixed-end contraction as a function of the initial sarcomere length. Sarcomeres
which begin at very large or small sarcomere lengths do not
shorten as much as sarcomeres of intermediate length. Explanation is given in text.
0
07,
20
I,
22
I.
24
I.
ze
I,
28
4.
30
.
I..
32
1.0
I
34
Muscle-Tendon Length (mm)
Fig. 8. Muscle-tendon length-tension curve for a fiber in
series with a perfectly stiff tendon (solid line) or a compliant
tendon (dotted curve) as measured in the current study. The
effect of tendon compliance is to increase the muscle-operating range. This is because a greater range of sarcomere
lengths is allowed to shorten ‘onto’ the descending limb of the
length tension curve.
1.5
2.0
2.5
3.0
3.5
40
Initial Sarcomere Length (pm)
Fig. 9. (A) Sarcomere length change during fixed-end contractions as a function of the initial sarcomere length. Different curves are shown for different connective-tissue length/fiber length ratios, varying from 1 to 15 (shown above curves).
As the ratio increases, a greater magnitude of sarcomere
shortening is permitted. (B) Sarcomere length-tension curves
corresponding to conditions shown in (A). Note that as the
ratio increased, the length-tension relationship is narrowed
and skewed to the right.
Model of frog muscle-tendon interactions
421
Fig. 10. Sample experimental record of the error associated
with the measurement of maximum tetanic tension in a
muscle-tendon unit. As contractile tension develops and
muscles shortening at a lower velocity is that they
maintain a greater relative tension (Cans and deVree,
1987).
A second consequence of tendon compliance is that
the operating range of the muscle-tendon
unit is
increased above that which would be predicted based
on the additive excursion of a number of sarcomeres
which are in series. Tendon compliance allows sarcomeres to shorten ‘into’ the length range at which
they can develop tension. This effect is especially
pronounced for muscles with very long tendons and
short muscle fibers such as the medial gastrocnemius
(Sacks and Roy, 1982) or flexor carpi ulnaris (Lieber et
al., 1990). This effect would probably be physiologically significant since the in viva frog ST sarcomere
length at the end of the swimming stroke was reported
to be 2.1 pm (Tidball and Daniel, 1986) and at the end
of the hop to be 2.6 pm (Mai and Lieber, 1990).
A final consequence of tendon compliance actually
represents a warning to physiologists in evaluating
skeletal muscle contractile components. Because compliant tendons allow muscles to shorten, they allow
muscles to decrease their internal passive tension
during shortening. As a result, the traditional method
for the estimation of maximum tetanic tension may
yield misleading information, the magnitude of which
will depend on the tendon length and material properties. The traditional experiment is modelled in Fig. 10.
Note that at sarcomere length 3.3 pm, some passive
tension exists (P,,,) before the muscle is activated. As
the muscle is activated it develops a certain ‘total
tension P,,,. This total tension, which represents the
sum of the active and passive tensions is used to
determine the active tension (P,) according to the
relationship P, = P,,, - PPss. However, there is a flaw
in this logic. The flaw is that P,,, is not constant
during the contraction, but actually decreases according to the passive sarcomere length-tension relation
(cf. Fig. 5). As a result, P, is underestimated since Ppas
overestimated
(Fig.
10). The
resulting
was
length-tension curve may be different from the actual
length tension curve of the muscle’s contractile component. The magnitude of the error depends on the
amount and material properties of the connective
tissue. As Zajac (1989) described, a useful parameter
for characterization of the muscle-tendon unit is the
muscle fiber length : tendon length ratio. In mammalian systems, this ratio can vary from about 1 to
about 15 (Zajac, 1989). ‘Compliant’ muscle-tendon
units can be considered those with high ratios while
‘stiff’ units are those with low ratios. The frog STtendon unit has a value of 1.5 for this ratio and is,
therefore, considered stiff. As the relative amount of
the connective tissue increases, the relative distortion
of the sarcomere properties occurs.
sarcomeres shorten, internal muscle passive tension decreases (solid line at the bottom). However, if the passive
tension is taken as the initial passive tension, active tension
will be underestimated (open arrows) compared to the actual
active tension (filled arrows). Simulation for sarcomere
length = 3.3 pm and initial passive tension level = 13%P,.
Acknowledgements-The authors thank Dr Felix Zajac and
Scott Delp (Stanford University) for helpful discussions on
different aspects of this project. This work was supported by
the Veterans Administration and NIH grants AR35192 and
DISCUSSION
The main result of this modelling exercise was that
the addition of a series compliance to a muscle, imparted properties to the muscle-tendon unit which were
unique from those predicted based only on the properties of the sarcomere. These results are qualitatively
similar to those predicted by Zajac (1989) and demonstrate that the relatively small but significant compliance present in the ST muscle-tendon unit has a
functional significance. The assumptions of the model
appear to be minimal. The assumption-that the muscle
velocity at continuously changing force can be described by the force-velocity relationship has recently
been confirmed in single fibers by Iwamoto et al.
(1990).
Numerous putative properties for tendon have been
proposed from the simple transmission of muscle force
to bones to the more complex storage of elastic strain
energy. Several recent experimental studies have suggested that muscle-tendon units must be considered
as an entity if an appropriate description of locomotion events is to be obtained. For example, Hoffer et al.
(1989) reported that during level walking in cats,
tendons lengthen considerabfy while muscle fibers
may actually shorten! Because of the asymmetry in
muscle mechanical properties (Katz, 1939) this phenomenon has important functional consequences.
First, it allows the tendon to absorb much of the
length change which accompanies ankle flexion. This
allows the muscle fibers to remain nearly isometric, or
even shorten during this yield phase. Thus, the very
high tensions, which are associated with muscle fiber
lengthening, are avoided. In a similar way, depending
on the tendon length and material properties, as the
muscle-tendon unit shortens, the tendon can actually
recoil, which permits the muscle fibers to shorten at a
lower velocity. It is well known that the advantage of
50
150
250
Time (ms)
428
R. L. LnZBER
AR40050. The authors thank Dr Zajac for providing an
advance copy of his review article. We also thank Margot
Leonard for constructive comments on the manuscript.
REFERENCES
Edman, K. A. P. (1979) The velocity of unloaded shortening
and its relation to sarcomere length and isometric force in
vertebrate muscle fibers. J. Physiol. 246, 255-275.
Ford, L. E., Huxley, A. F. and Simmons, R. M. (1977) Tension
responses to sudden length change in stimulated frog
muscle fibres near slack length. J. Physiol. 269, 441-515.
Ford, L. E., Huxley, A. F. and Simmons, R. M. (1980) The
relation between stiffness and filament overlap in stimulated frog muscle fibers. J. Physiol. 311, 219-249.
Gans, C. and deVree, F. (1987) Functional bases of fiber
length and angulation in muscle. J. Morphol, 192, 63-85.
Gordon, A. M., Huxley, A. F. and Julian F. J. (1966) The
variation in isometric tension with sarcomere length in
vertebrate muscle fibers. J. Physiol. 184, 170-192.
Hatze, H. (1973) A theory of contraction and a mathematical
model of striated muscle. J. theoret. Biol. 40, 219-246.
Hoffer, J. A., Caputi, A. A., Pose, I. E. and Griffiths, R. I.
(1989) Roles ofmuscle activity and load on the relationship
between muscle spindle length and whole muscle length in
the freely walking cat. Prog. Brain Res. 80, 75-85.
Huxley, A. F. (1957) Muscle structure and theories of contraction. Prog. Biophys. 7, 255-318.
Iwamoto, H., Sugaya, R. and Sugi, H. (1990) Force-velocity
relation of frog skeletal muscle fibers under continuously
changing load: J. Physiol. 422, 185-202.
Julian. F. J. and Morean. D. L. (1981) Tension. stiffness.
unloaded shortening speed and potentiation of frog muscle
fibres at sarcomere lengths below optimum. J. Physiol.
319,205-217.
Katz, B. (1939) The relation between force and speed in
muscular contraction. J. Physiol. 96, 45-64.
Lieber, R. L. and Boakes, J. L. (1988) Sarcomere length and
joint kinematics during torque production in the frog
hindlimb. Am. J. Physiol. 254, C759-C768.
Lieber, R. L. and Leonard, M. E. (1989) Measurement of frog
et al.
semitendinosus force, tendon load-deformation
and
load-strain properties. J. Biomechanics 22, 1048.
Lieber, R. L., Fazeli, B. M. and Botte, M. J. (1990) Architecture of selected wrist flexor and extensor muscles. J. Hand
Surg. 15, 244250.
Lieber, R. L., Leonard, M. E., Brown, C. G. and Trestik, C. L.
(1991) Frog semitendinosis tendon load-strain
and
stress-strain properties during passive loading. Am. J.
Physiol. 261, C86C92.
Mai. M. T. and Lieber. R. L. (1990) Interaction between
semitendinosus muscle and knee and hip joints during
torque production in the frog hindlimb. J. Biomechanics
23,271-279.
Morgan, D. L. (1976) Separation of active and passive
components of short-range stiffness of muscle. Am. J.
Physiol. 232, C45-C49.
Morgan, D. L., Mochon, S. and Julian, F. J. (1982) A
quantitative model of intersarcomere dynamics during
fixed-end contractions of single frog muscle fibers. Biophys.
J. 39, 189- 196.
Rack, P. M. H. and Westbury, D. R. (1984) Elastic properties
of the cat soleus tendon and their functional importance. J.
Physiol. 347,479-495.
Ramsey, R. W. and Street, S. F. (1940). The isometric
length-tension diagram of isolated skeletal muscle fibers of
the frog. J. cell. camp. Physiol. 15, 11-34.
Sacks, R. D. and Roy, R. R. (1982) Architecture of hindlimb
muscles of cats: functional significance. J. Morphol. 173,
185-195.
Schmidt-Nielsen, K. (1984) Scaling-Why is Animal Size So
Important? Cambridge University Press, Cambridge, England.
Squire, J. M. (1990) (Editor) Molecular Mechanisms in
Muscular Contraction. CRCPress, Boca Raton, FL.
Tidball. J. G. and Daniel. T. L. (1986) Elastic enerev storage
in rigored skeletal muscle cells under physiological loading
conditions. Am. J. Physiol. 250, R56-R64.
Walmsley, B. and Proske, U. (1981) Comparison of stiffness
of soleus and medial gastrocnemius muscles in cats. J.
Neurophys. 46, 250-259.
Zajac, F. E. (1989) Muscle and tendon: properties, models,
scaling and application to biomechanics and motor control. In CRC Critical Reviews in Biomedical Engineering
(Edited by Bourne, J. R.), Vol. 17, pp. 359411, CRC Press,
Boca Raton, FL.