General Chemistry I Exam 3 - Fall 2010
advertisement
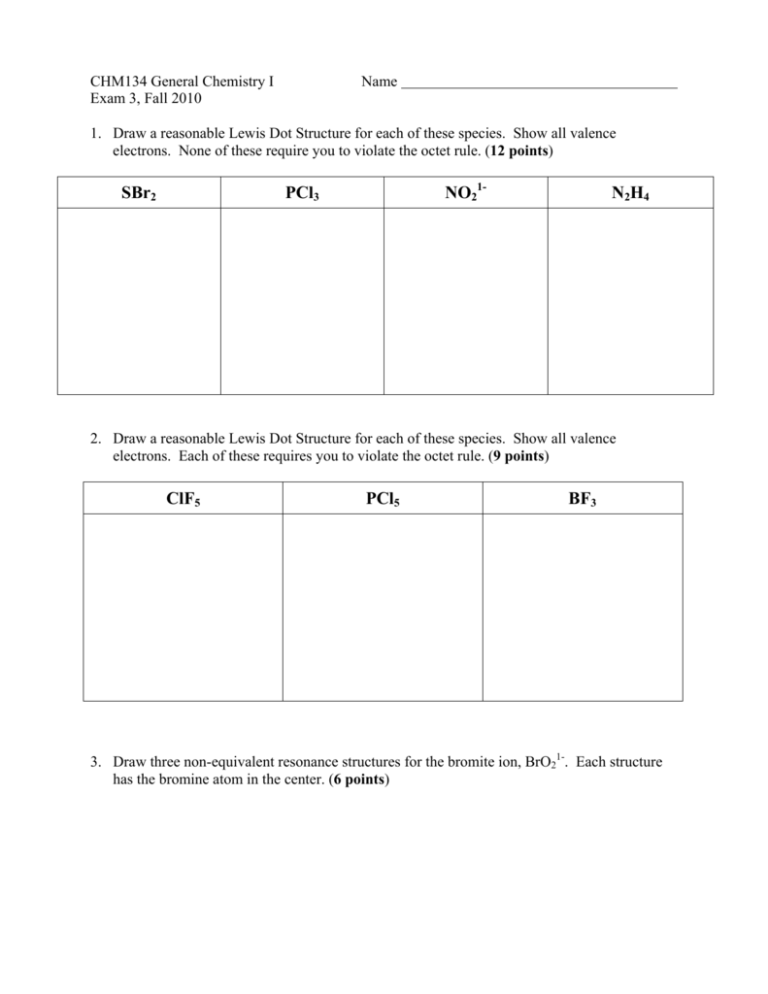
CHM134 General Chemistry I Exam 3, Fall 2010 Name 1. Draw a reasonable Lewis Dot Structure for each of these species. Show all valence electrons. None of these require you to violate the octet rule. (12 points) SBr2 NO21- PCl3 N2H4 2. Draw a reasonable Lewis Dot Structure for each of these species. Show all valence electrons. Each of these requires you to violate the octet rule. (9 points) ClF5 PCl5 BF3 3. Draw three non-equivalent resonance structures for the bromite ion, BrO21-. Each structure has the bromine atom in the center. (6 points) 4. Based on the Lewis Structures provided, complete the table below by providing a sketch of the species (in 3-D, if appropriate), supplying the idealized bond angles, naming the molecular geometry of the species, identifying the type of orbital hybridization present on the central atom of the species, and indicating whether the species is polar or nonpolar. (20 points) Molecule SO2 SeBr4 NH3 SiF4 Lewis Structure Sketch (with angles) Geometry Hybridization on central atom Polarity 5. Determine whether a bond between each of the following pairs of atoms would be nonpolar covalent, polar covalent, or ionic. (4 points) Li and O C and O N and S Cl and Cl 6. Assign formal charges to the elements in each of the structures below. Based on your assigned formal charges, circle the structure that makes the greatest contribution to the correct structure of the species. (7 points) H H C H S H S C H C S 7. Use bond energies from the table provided to determine the enthalpy change for the following reaction. (8 points) O O N O + 4 H2 N O N2 + 4 H2O 8. Consider the structures of caffeine (left) and vitamin C (right) below in order to answer the questions that follow. (3 points each, 15 points total) a. How many sigma (σ) bonds are present in the vitamin C structure? b. How many pi (π) bonds are present in the caffeine structure? c. How many sp2 hybridized carbon atoms are present in the caffeine structure? d. How many sp3 hybridized oxygen atoms are present in the vitamin C structure? e. How many sp2 hybridized nitrogen atoms are present in the caffeine structure? 9. For each of the pairs of orbitals shown below, considering their types and orientation, what type of bond is the most likely to form? (4 points) Use the following molecular orbital diagram to help answer the remaining questions. (10 – 12 are worth 3 points each; 13 – 15 are worth 2 points each) 10. According to molecular orbital theory, what is the bond order in the oxygen molecule? 11. According to molecular orbital theory, is the oxygen molecule diamagnetic or paramagnetic? 12. According to molecular orbital theory, what is the bond order in the F21- ion? 13. According to molecular orbital theory, which species below is least likely to exist? F22+ F2 F22- 14. According to molecular orbital theory, which of these species has the longest bond length? O22+ O2 O22- 15. According to molecular orbital theory, which of these species will be attracted to a magnetic field? F22+ F2 F22-