Aspirin – Part I II & III: Synthesis, Quantitative & Qualitative Analysis
advertisement

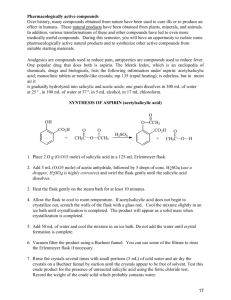
Aspirin – Part I II & III: Synthesis, Quantitative & Qualitative Analysis March 30, 2011 Matt Smith Bradley LaBoon Introduction: Aspirin holds a history that dates back to the days of Hippocrates, where he left medical records of using powder from the willow bark to ease headaches, pains and fevers. According to Nick Henderson, Executive Director of the Aspirin Foundation (2009), it was not for years until the salicin within this willow bark was found to be the ingredient to ease such pain. Many scientists extracted this salicin over the years until it was titled the name salicylic acid, in its pure form. It wasn’t until 1900 that aspirin was marketed by Felix Hoffmann who, at the time, worked for Bayer. At first, aspirin was sold as a powder, where as in 1915, the marketed drug switched to a tablet (p.12). This drug has become a valued medical innovation with the help of the salicylic acid ingredient. Aspirin has been found to treat more than just pain. According to Tash Hughes, of Word Constructions, the drug has been proven to reduce swelling/inflammation, reduce the severity of a heat attack, aid recovery after cardiovascular surgery, and even treatment of several rheumatoid diseases (p.23). Many more uses are tested daily for success, as the salicylic acid within the drug proves to aid in more than just headache relief. Due to the fact, however, that aspirin is considered an analgesic, there are possible stomach upsets, and very slight kidney problems. Despite these side effects, aspirin proves to work wonders in the medical field. Purpose: The purpose of this experiment was to first complete an esterification reaction with salicylic acid and acetic anhydride to produce acetylsalicylic acid in order to observe the purity of an aspirin sample. Several samples will be produced using varied ingredients in order to examine the purity of each, noting how each differs from the commercially produced aspirin samples. From here, several samples of given aspirin will react with an iron (III) chloride solution to determine the purity of the ‘raw’ and re-crystallized experimental samples produced in the previous step. The two commercial aspirin samples will also be observed for purity and the results will be compared to each other. Method/Procedure: In Part I of the experiment, salicylic acid and acetic anhydride were reacted to produce acetylsalicylic acid, or in other words, aspirin: To begin this, a 6-gram sample of salicylic acid was recorded and combined with 12 mL of acetic anhydride along with a few drops of sulfuric acid. The reagents were mixed thoroughly and placed in a hot water bath for a time for 20 minutes. From here, the heated reaction solution was allowed to cool to room temperature, and 50 mL of distilled water was added to form crystals before going in an ice bath. The solution then underwent a vacuum filtration in order to separate the formed crystals from the liquid. The crystals were let to dry and ground up before half were placed into a vile labeled ‘raw.’ This is when recrystallization occurs in order to improve the purity of the aspirin sample. The rest of the raw crystals were then dissolved with small amounts of ethanol and then placed in the hot water bath again. Here, the dissolved crystals run through the same crystallization procedure as before. However, this process is not rushed, as a good recrystallization takes time to produce a high quality product. Once finished, these crystals are placed into a vile labeled ‘pure.’ In Part II, a series of three tests were taken to provide information about the presence of impurities. The samples synthesized in Part I, were likely to contain small amounts of impurities, including less in the recrystallized sample. To test other samples, a TLC analysis was taken. Marks were made along the top and bottom of the TLC plate along with 5 marks on the bottom for each of the 5 samples (salicylic acid, new commercial aspirin, old aspirin, raw aspirin, & recrystallized aspirin). A few crystals of salicylic acid were placed on a watch glass along with <1 mL of ethanol. Using a micropipette, a spot was placed on the TLC plate, along the bottom. This spotting process was repeated for each sample and allowed to dry on the plate. A small amount of eluent solution was placed in a beaker along with the TLC plate and covered until the solution reached the end line. The positions of each spot was observed under black light and recorded. For a second test, five small amounts of each sample were placed in 5 test tubes, each including 5 mL of ethanol and a few drops of 1% ferric chloride solution. The colors were observed, with a purple color indicating the presence of salicylic acid. For the final test, melting point tubes were prepared for each sample and placed in the apparatus. The melting points were observed and recorded, looking for a range of temperatures to indicate purity. In Part III, the purity of the aspirin sample was determined through a quantitative version of the iron (III) chloride test already performed. A series of absorbances of known concentrations of salicylic acid can be compared to the absorbance of the experimental samples. To begin, a small sample (.12g - .15g) of salicylic acid was weighed and placed into a flask and dissolved with 10 mL of ethanol. This was diluted to the 100 mL line with distilled water and mixed thoroughly, which acted as the stock solution. A series of solutions was prepared using indicated volumes of stock solution and diluted with a 0.02 M iron (III) chloride solution to a certain total volume and mixed. To prepare the unknown solutions, 0.15 grams of each sample (raw, recrystallized, new store-bought aspirin, & old store-bought aspirin) were placed in separate 100 mL volumetric flasks. 10 mL of ethanol was added to each to dissolve the sample and diluted to the mark with distilled water. 5.00 mL of this solution was place in a 50 mL flask and then diluted with the iron (III) chloride solution used earlier. These 8 solutions were then placed into separate cuvettes, about 2/3 full. Using a ‘blank’ of the iron (III) chloride solution, the spectrometer was set using six parameters. After running with the first solution, the wavelength and absorbance of the peak on the scan was recorded and entered before running each of the cuvettes. Part I: Mass of salicylic acid Mass of sample Raw observations Pure observations 6.330g 7.195g One, moist clump of off-white material Many pure, white crystals that appear dry in powder form Part II: Sample Range Raw Pure Old Aspirin New Aspirin Salicylic Acid Qualitative Observations Dark purple Medium shade purple Light BurntOrange/Yellow Light BurntOrange/Yellow Deep purple Melting Point 121.0-123.9 129.4-134.0 133.5-134.8 131.0-134.4 158.9-160.2 Data for Preparation of Standard Solutions Mass of salicylic acid 0.145g Volume of Stock Solution Prepared 100.00 mL Standard Solution Volume of Stock Solution Used (mL) Final Volume of Standard Solution (mL) Absorbance A 5.00mL 50.00mL 1.086 B 5.00mL 100.00mL 0.589 C 1.00mL 50.00mL 0.212 D 1.00mL 100.00mL 0.133 Calculations EQUATION EXAMPLE !"## !"#$%&#$% !"#$ !"#$% !"## Moles salicylic acid Molarity of undiluted standard solution !. !"#$ ! !"#. !" !"# = !. !!"!#$% !"#$%#&'(#&'%'&) . !""# !. !!"!#$%& . !""# = !. !"# ! ! ! !! = ! ! !! . !"#$ ∗ . !!" = !! ∗ . !"# !! = !. !!"#$ Molarity of diluted standard solution Results Solution Concentration A 0.0015M B 0.00075M C 0.0003M D 0.00015M Graph of absorbance vs. concentration (including trend line): 1.2 1 0.8 y = 717.28x + 0.0208 R² = 0.99714 0.6 Series1 Linear (Series1) 0.4 0.2 0 0 0.0005 0.001 0.0015 0.002 Data for Unknown Solutions Sample Sample Mass Solution Absorbance Raw Experimental Aspirin 0.153 g 1.190 Recrystallized Experimental Aspirin 0.160 g 0.369 Commercial Aspirin – New 0.150 g 0.024 Commercial Aspirin – Old 0.147 g 0.239 Calculations – using Raw Experimental Aspirin as the example EQUATION !−! ! !. !"#−. !"!# = !. !!"#$ !"!. !" !"#$% !" !"#$%& !"#$%& !" !"#$%&"' !. !!"#$ ∗. !"" ! !"#$%&"' = !. ! ∗ !"!! Molarity of salicylic acid in solution != Moles of salicylic acid in solution != Mass of salicylic acid in unknown sample !"#$% = Mass of aspirin present in unknown sample Percent purity of aspirin in unknown sample EXAMPLE !"## !"#$%&#$% !"#$ !"#$% !"## !"## !! !"#$%& − !"## !" !"#$%&#$% !"#$ !"## !" !"#$%& − !"## !" !"#$%&#$% !"#$ !"##$%#"!&'( ∗ !"" !. ! ∗ !"!! ∗ !"#. !" 0.0221g ! = !"# !. !"#$ − !. !""#$ = !. !"!# !. !"#!−. !""#$ = !". !% !. !"#$ Results Sample Solution Concentration Mass of Salicylic Acid Mass of Aspirin Purity Raw Exp. Aspirin 0.0016M 0.0221g 0.1310g 85.6% Recrystallized Exp. Aspirin 4.85 ∗ 10!! 0.0067g 0.1531g 95.8% New Aspirin 4.46 ∗ 10!! 6.16 ∗ 10!! 0.1499g 99.9% Old Aspirin 3.04 ∗ 10!! 0.0042g 0.1428g 97.1% Discussion: The purpose of this lab was to observe and analyze the synthesis reaction of aspirin. To start out the lab, salicylic acid became the main component of the reaction as it was added with acetic anhydride. This reaction went fairly smoothly, producing decent samples of both raw and pure aspirin. The ‘raw’ sample, however, seemed to retain more water than the other sample and was more of a ‘gummy’ texture. Through recrystallization of the aspirin, the pure sample turned out to be more ‘powder-like’ and much whiter in color. Once the samples were made, the tests to analyze them began. First, was the iron (III) chloride test in which each sample was combined with the iron solution to observe the color produced. The presence of salicylic acid creates a dark purple color, varying from sample to sample based on the amount contained. The raw and recrystallized samples both produce a relatively dark purple color, very different from the burnt orange colors of the store bought aspirins. The raw should have contained amounts of salicylic acid within the sample, although the recrystallized sample should have contained a smaller amount. This could have been due to a faulty recrystallization and/or the contamination of the samples. A vacuum filtration was used to filter out the crystals from the solution, which could have not completely filtered correctly. If the process was rushed, and the crystals were not let to dry long enough, the samples may have retained any bit of water and/or salicylic acid. The TLC plate reading proved to be very temperamental as our plate was unreadable. This could have been due to the extended amount of time the plate was left in the eluent solution. This produced a dark line of color at the top of the plate, proving to be useless. However, another TLC was provided for qualitative reasons about the 5 different solutions. From here, the melting point test went very smoothly as the steps to the procedure were minimal. All 5 samples exhibited melting point ranges that fell well within the standard ranges. In Part III, a stock solution was prepared to be used for the rest of the experiment as it contained the iron (III) chloride and salicylic acid. Since the salicylic acid was measured on a non-exact basis, the amounts of solute differed from group to group-producing slightly different results. Although the amount massed out fell within the right amount needed to create the perfect solution. The process of creating both the standard solutions and the unknown solutions did not seem to have any obvious problems, as the instructions guided the process along smoothly. Once read in the spectrometer, each cuvette of the standard solutions proved to have a correct absorbance according to the actual readings given. When it came to the unknown samples, the absorbance readings were much different and had a negative value for each of the four samples. This occurrence may have been due to the over dilution of the samples. Within the procedure, the process calls for a second dilution after the first, in order to clear up the color of the solution. This extra process may have washed out the color completely of each, resulting in a lower absorbance value than the iron (III) chloride ‘blank’ solution. In terms of qualitative tests, this experiment showed many varying results. In the iron chloride test, small amounts of ethanol dissolve each of the samples and then combined with a few drops of 1% ferric chloride. Since the salicylic acid will react with the iron (III) chloride solution, a distinct purple color was apparent in the salicylic acid test tube. The new and old aspirin samples went along with this as they produced a light orange/yellow color, showing the absence of salicylic acid within the solutions. This tells us that the store bought aspirins are relatively pure, and do not contain large amounts of salicylic acid. However, the raw and pure samples both produced a dark purple color, indicating the strong presence of salicylic acid. This should not have come out with such a dark color in either of the two solutions. While the raw sample would have some trace amounts of the acid, the pure should have gotten rid of those through recrystallization. This means the process was not a success. In turn, this means that both the raw and pure samples have a low percent of purity. In the TLC plate analysis, our plate came up with a long, dark band across the top. This means the bands had all dissolved and ran to the top of the plate, providing no understandable results. The time left in the solution must have been too long for this to happen. When observing the given TLC plate however, the salicylic acid column contains no band but only a large spot at the top. This suggests that the salicylic acid was fully dissolved into the solution and was able to run to the end of the plate. When it came to the new store bought aspirin, there was no spot at the top. This should have been the most pure out of all the samples, which shows that there was not trace of salicylic acid within the sample placed. Each spot there after, from left to right, grows increasingly bigger. This shows the presence of salicylic acid increases as you go from new to old to recrystallized to raw samples. Finally, the melting point test showed us that the samples we had produced or were given seemed to be very precise. Each sample melting under the heat fell within the normal ranges of each sample. This proves the identity of each sample as it was given. All in all, the qualitative tests had several difficulties but seemed to show vital information. While the melting point test proved to be correct, the other two tests were not consistent. This questions the purity of the raw and pure samples made in Part I. In terms of the quantitative tests, the results seemed to be consistent in terms of purity. The standard solutions created with the given values in the table seemed to give accurate absorbance values. As they were produced on the day of the Part III experiment, they did not have any dealings with the other samples made in previous weeks. They simply acted as a gauge to what the absorbances should have looked like in the end. The unknown solutions, however, produced negative absorbance values. When observing these cuvettes before placement into the spectrometer, the colors all seemed to be extremely washed out. After going back to the lab manual and rereading the procedure, this could have been due to the second diluting stage of the process. The experiment calls for a second dilution with distilled water. After it was once diluted with the iron chloride solution, the distilled water would have most definitely washed out any color previous to this process. When compared to the blank solution, the color would be much less, creating a negative absorbance value. While this does not provide much insight into the problems of Part II, it remains consistent with the faulty misreading’s of the created solutions. After examination, I would say the second week provided the most insight into the reliability of my samples. One would imagine the quantitative tests to provide the most information about an experiment, however the qualitative results provided direct results able to be compared to one another. Seeing the dark colors of the produced raw and recrystallized samples proved the presence of salicylic acid in a direct way. In week three, the actual procedure proved to be faulty, as the second dilution process washed out the colors of all samples. If this had not been true, the third week would have provided concrete evidence to the reliability of the samples using absorbance. References: Henderson, Nick. "What Is Aspirin - 100 Years of Aspirin." Aspirin Foundation. Mar. 2009. Web. 30 Mar. 2011. <http://www.aspirin-foundation.com/what/100.html>. Hughes, Tash. "Aspirin Use Article." Word Constructions Writing Services. 23 Apr. 2003. Web. 30 Mar. 2011. <http://www.wordconstructions.com/articles/health/aspirin.html>.