The University of Michigan Experience to Date with Advanced Asset
advertisement

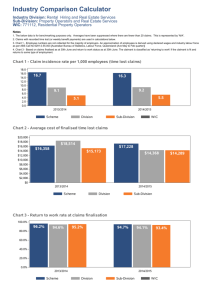
Innovation in the LIS: Implications for Design, Procurement and Management Ulysses J. Balis, M.D. Director, Division of Pathology Informatics & Director, Pathology Informatics Fellowship Program Department of Pathology University of Michigan ulysses@med.umich.edu Pathology Informatics 2012 Meeting: October 9, 2012 Chicago Illinois An Evolving Tension: EHR vs. LIS • Thesis Statement: – The contemporary stand-alone LIS model is being challenged by the reality of fullyintegrated primary vendor EHR solutions, which promise to provide functional equivalence of the stand-alone LIS with the added benefits of: • • • • • • Simplified enterprise complexity Simplified deployment logistics Reduced total cost of ownership Simplified long-term stewardship of both software, hardware and data Enhanced patient safety Simplified Meaningful Use certification • Justification: – If the pathology -owned LIS can be justified as the correct place to house data and workflow in the overall enterprise data model, Pathology will be allowed to continue in its role of being elector and steward of such systems Key LIS Innovation Areas to Consider • Enterprise Data Models and Support for High Dimensional Data • Shared Stewardship of Knowledge Management Systems • Reverse federation in support of personalized medicine (bidirectional data feeds) • Intrinsic support for customized workflow – “Workflow Engines” • Transition to zero knowledge interfaces (XML) • LIS interoperability • Digital Pathology Workflow Lee Hood, IOM February 27, 2012 Challenge: Evolving the contemporary LIS will be critical to staying current with the anticipated deluge of knowledge management challenges facing the clinical laboratory Population(s) From: Athey and Omenn, 2010 The Clinical Laboratory Information System holds the potential to be the “Information Commons” for all aspects of P4-assoicated patient data. George Poste, IOM Feb. 28, 2012 Lee Hood IOM February 27, 2012 Five _____ Education and Training View ‘Omics Enhanced William S. Dalton; Moffitt Cancer Center; IOM Feb. 27, 2012 Whole Genome Mapping and Variant Annotation Pipeline: an EHR or LIS functional component? Genome Mapping and Raw Output Pre-Processing Input Map Algorithm 1 Output Result 1 Output Custom Conversion Script Map Algorithm 2 Output Result 2 Output Custom Conversion Script Mapping Algorithms Pre-Processed Variant Data D. Wall, CBMI, HMS PreProcessed Data Custom Conversion Script Standardization, Annotation, and Summary of Results Standardized Variant Output File Annotate Variants and Analyze Quality and Coverage Mapping & Annotation Summary Report Finalized Variant Output Files (HGVS) From WGA to Clinical Annotation: Possible LIS workflow Paired-end WGA workup sequencing ordered RNA-seq Database Oncogene/Tumor Suppressor Detection Validation (FISH, RTPCR, Sanger) Validation Gene Expression Treatment Plan Prepared Amplified CNV Over-expressed Deleterious, LOH Variants Identified FDA Approved On-Label FDA Approved Off-Label Clinical Trial Underway Medical Impact Report Generated D. Wall, CBMI, HMS, Athey & Omenn Global Presence of an LIS-centric world view at the enterprise level:: Education, Research, & Patient Care Brian Athey & ECRIT 1/11/11 Admissions Clinical Scheduling & Grading System Education IT Security IT SERCUIRTY Research Pre, Post- Award Bioinformatics Research Click Administration Commerce Systems (IRB) Research Proteomics Core Metabolomics Facilities/ ‘Omics’ Ctools/Saki 3 eThority (billing) Tissue Biorepositories Visiting Student Application Service(VSAS) M-Pathways Collexis ULAM Education Knowledge Repository Research Administration Data Warehouse RedCAP Populations Research Research & BioDBX Individuals Data Quality Diseases Velos Management Metrics Systems Data Marts Demographics OpenClinica Clinical Quality Analysis Metrics Database Reporting (CAD) & Peer Others Review Others … Registries Research Data Warehouse CareLink/ Eclipsys Emergency Med. Pharmacy Cycle Patient Care Revenue Systems Pathology Legacy+/Epic EHR Radiology Scheduling HIM/ Documentation Others… CDR Epic Clarity HSDW Enterprise Federated Data Warehouse CAD Historical SPORES i2b2 Ambulatory Data Biomedical Engineering HIPAA/IRB Services (Honest Broker, DE-ID Consent Management, …) Common Identifier Services (Patient, Provider, Information Research, Specimens, Service-Oriented BusExternal Mappings) Vocabulary & Terminology Mapping Services (ICD-9/10 SNOMED, IMO, caDSR, ...) Security ITITSecurity Campus Systems Curriculum Eval. System Next-Gen Sequencing Portals / Providers, Payors, P. Health Databases / HIEs / NHIN Comprehensive Clinical Assessment Exam Messaging Bus, ETL & External Collaboration Services (SOA, caGRID, SHRINE, ...) Health Sciences Library Resources NIH-Specific & External Data Resources (PubMed, GenBank, KEGG, GO, etc.) High Performance Cloud Computing & Data Storage Bioinformatics and Systems Biology Workbenches • Reporting • Visualization • Analysis & • Data Mining Data Sharing with External Collaborators International Industry: Pharma/ caBIG I2b2/ CTSAs Biotech TCGA SHRINE Global Presence of an LIS-centric world view at the enterprise level:: Education, Research, & Patient Care Education Admissions Clinical Scheduling Metabolomics BioDBX Individuals M-Pathways Tissue Biorepositories Diseases eThority (billing) Velos Others… Collexis ULAM CTools/Sakai 3 IT Security IT SERCUIRTY Campus Systems Curriculum Evaluation System Education Knowledge Repository Research Administration Data Warehouse Populations CIDSS Analytics & Reporting Tools OpenClinica Demographics Registries Others … Research Data Warehouse i2b2 Historical Data Ambulatory Emergency Med. Pharmacy Pathology Revenue Cycle Radiology Scheduling Centricity Documentation Others… CDR HSDW CAD SPORES Others CareLink/ Eclipsys HIM Security ITITSecurity Click Commerce (IRB) Proteomics RedCAP Patient Care Systems Legacy + Epic Epic EHR Epic Clarity Biomedical Engineering HIPAA/IRB Services (Honest Broker, De-ID Consent Management, …) Common Identifier Services (Patient, Provider, Research, Specimens, External Mappings) Vocabulary & Terminology Mapping Services (ICD-9/10 SNOMED, IMO, caDSR, ...) Portals / Providers, Payors, P. Health Databases / HIEs / NHIN Comprehensive Clinical Assessment Exam Research Research Core AdministrationFacilities/‘Omics’ Quality Systems Metrics Research & Research Reporting Quality Metrics Data Next-Gen & Management Data Marts Sequencing Research Pre, Peer Systems Post- Award Bioinformatics Review Brian Athey & ECRIT 1/11/11 Messaging Bus, ETL & External Collaboration Services (SOA, caGRID, SHRINE, ...) Health Sciences Library Resources NIH-Specific & External Data Resources (PubMed, GenBank, KEGG, GO, etc.) High Performance Cloud Computing & Data Storage Bioinformatics and Systems Biology Workbenches • Reporting • Visualization • Analysis & • Data Mining Data Sharing with External Collaborators International Industry: Pharma/ caBIG I2b2/ CTSAs Biotech TCGA SHRINE Concept: Reverse Federation • Not only does the LIS serve the greater EHR connectivity ecosystem, so to should the greater plurality of EHR repositories serve the LIS and its emerging workflow needs, in the capacity of decision support data feeds assisting in report generation. – Increasingly essential for: • Molecular reporting • Personalized medicine • Synoptic cancer checklist reporting of correlation of histopathology staging in concert with clinical stage and longitudinal reporting (AJCC Cancer Staging Manual, 7th edition and later…) Concept: Complex data & Thiopurine Metabolite testing: The emergence of Encoded Data from primary lab test data Comparing MLA to 6-TGN Summary • The future LIS will leverage: – Enterprise Data Models and Support for High Dimensional Data – Shared Stewardship of Knowledge Management Systems – Reverse federation in support of personalized medicine (bi-directional data feeds) – Intrinsic support for customized workflow • “Workflow Engines” – Transition to zero knowledge interfaces (XML) – LIS interoperability – Digital Pathology Workflow