CHAPTER 5 Microbial Metabolism
advertisement

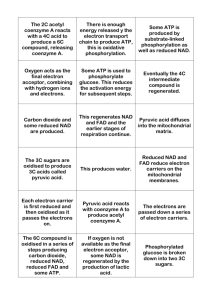
270 13. 10. 27. 18. 7. 9. 8. 21. 19.for B D C AB A B 14. 15. 12. 11. 25. 26. 16. 17. 20. 22. 23. 24. C C D D CMicrobiology Study Guide 1. B Multiple Choice CHAPTER 5 Microbial Metabolism Fill in the Blanks 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. precursor metabolites, energy, exergonic, release donors, oxidation, acceptor, reduction NAD+, NADP+, FAD competitive, noncompetitive, competitive respiration, fermentation pentose phosphate pathway, Entner-Dourdoroff pathway, pyruvic acid answers are as follows: 1) glycolysis: cytosol, cytosol; 2) Krebs cycle: cytosol, mitochonrial matrix; 3) Electron transport chain: cytoplasmic membrane, inner mitochondrial membrane; and 4) photosynthesis: cytoplasmic membrane invaginations called thylakoids, invaginations of the inner chloroplast membrane which form thylakoids protons, proton gradient, chemiosmosis oxygen, inorganic molecules proteases, deamination, Krebs cycle cyclic, PSI, noncyclic, PSI, PSII amphibolic Matching 1. 2. 3. 4. 5. A, B, C, D, G A, D, F A,E F B, C 6. 7. 8. 9. 10. D, G B,E B A, C, G A, B, C, D, G Short-Answer Questions for Thought and Review 1. Allosteric inhibition is a type of noncompetitive inhibition where regulatory molecules bind to enzymes at sites other than the active site and turn the enzyme off. The binding is irreversible and generally involves changing the shape of the enzyme so that it can no longer function (cannot bind substrate). Allosteric inhibition essentially "removes" enzymes from the mix, thus the only way to overcome the inhibition is to produce more enzyme above the level of the inhibitor. 2. Fermentation is necessary to regenerate electron carriers (i.e., NAD+) to keep glycolysis running. Cells that ferment can't use cellular respiration to do this and so the only way to keep making even small amounts of energy is to keep using glycolysis, which means you need to continually recycle the supply of NAD+. Answers to Study Guide Questions 271 3. Catabolism and anabolism are essentially two halves of the same coin. Catabolism breaks down molecules into pieces that anabolism uses to construct new macromolecules for the cell. If anabolism proceeded at a faster rate than catabolism, eventually the anabolic systems would run out of building blocks and the cell would stop synthesis. If the cell were attempting to divide, it wouldn't be able to because it couldn't produce all of the new parts it needed. 4. An example is given for step 3 of the Krebs cycle as shown in Figure 5.19. NAD+ is the oxidized form of the electron carrier, NADH is the reduced form. Isocitric acid and a-ketoglutaric acid are both Krebs cycle intermediates. In the equation below, the electron donor is the isocitric acid that loses COOH in its enzymatic conversion to a-ketoglutaric acid. The hydrogen atom is transferred to NAD+, the electron acceptor, which becomes reduced in the process. CO2 is evolved off essentially as waste. Overall, the conversion of isocitric acid to a-ketoglutaric acid is an oxidation reaction and the conversion of NAD+ to NADH is a reduction reaction. Isocitric acid + NAD+ -7 a-ketoglutaric acid + NADH + CO2 Critical Thinking 1. The advantage to synthesizing everything you need from a small number of precursors is that you are a bit less dependent on finding correct nutritional equivalents in the environment. Such an organism can live in an environment where nutrients are not readily available or where they appear in fits and starts. The disadvantages would include the fact that you will always be using a lot of energy to synthesize everything, and to some extent you will have a higher burden to keep many more metabolic pathways functioning normally. It's best to be able to do both because when nutrients are available, you can save some energy by importing materials rather than making them, but when the nutrients aren't there you won't starve because you can make what you need. In this way, you can survive the variability of the environment to a much greater extent than microbes that cannot switch metabolic functions. 2. There are a couple of ways the cell can control amphibolic pathways. In the case of glycolysis and gluconeogenesis, the key points where the pathway would become dedicated to catabolism or anabolism, respectively, are actually controlled by different enzymes. Thus if you want glucose, a different set of enzymes is activated than if you want to break down glucose. The pathways are controlled at the point of entry. Another way such pathways can be controlled is through the use of inhibitors and/or feedback type situations where the products of the pathway (both catabolic and anabolic) can be used to modulate the activity of enzymes in the pathway to direct the pathway in one direction or another depending on need. 3. In the oxygenated environment, cellular respiration using the electron transport chain will be the dominate energy generation mechanism. ATP is made primarily from the proton motive force generated by the electron transport chain. If the organism is suddenly moved to an anaerobic environment, respiration will stop once the last terminal electron acceptor (oxygen) is used up. At this point one of two things will happen. If the cell is capable of fermentation, it can continue to generate very small amounts 272 Study Guide for Microbiology of ATP from the continual cycling of glycolysis, using fermentation to regenerate NAD+. If the cell cannot ferment, and cannot use an alternate electron acceptor, then it will most likely die. Concept Building Questions 1. The simple answer as to why microbes can't import whole proteins inside rather than make them is that proteins are simply too large to pass through the outer structures microbes possess. Prokaryotes have complex cell walls that are breached only by special channels or pores. Other microbes have cytoplasmic membranes at the very least (some will also have other structures) that are also impassible by large proteins. Proteins cannot diffuse through a membrane, nor are the channels, pores, or other transporters large enough to allow such big molecules through. If they were, the holes would be so big in the membranes of microbes that everything else could pass in and out of the cell with little control and most likely the cell would burst because of its inability to control movement of small molecules. 2. Enzyme-substrate complexes will be formed via weak interactions between the two: hydrogen bonds, electrostatic associations, and some conformationally derived interactions. Essentially, those bonds that can be easily formed and reformed will be used in the enzyme-substrate complex. Covalent bonds of any sort cannot be used to establish this complex because they are not readily dispensable. A given enzyme may be needed to break a covalent bond in a substrate, but it is not going to be able to work on the substrate to the extent it needs to do that if it is itself permanently bound to the substrate. Thus, covalent associations would prevent the enzyme from moving the substrate, aligning it in the correct orientation, etc. In other words, function would cease even though the enzyme would be bound to the substrate. CHAPTER 6 Microbial Nutrition and Growth Multiple Choice 1. D 6. 7. 8. 9. D B D D 11. B 16. B 12. D 17. C 18. A 10. D 15. B 2. B 3. A 4. C 5. C Fill in the Blanks 1. nutrients 2. do, E. coli 3. nitrogen fixation 4. psychrophiles,45 5. acidophiles 6. broth, colonies 7. streak plate 8. enrichment 9. 6300+ 10. turbidity, indirect 13. A 14. D