Mauro's Nomenclature Cheat Sheet
advertisement

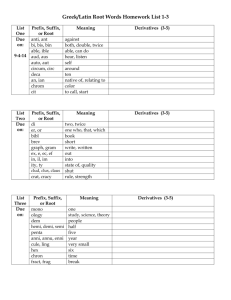
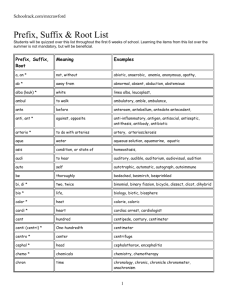
Mauro’s Nomenclature Cheat Sheet Two Non-Metals (Type III) (Prefix1) + 1st Element Name + Prefix + 2nd Element Root Name + “-ide” Prefixes: • Mono • Di • Tri • Tetra 1 • • • • Penta Hexa Hepta Octa • • Nona Deca If Prefix number of 1st Element Name is 1, Mono- isn’t used. Order: 1o Elements further left in the periodic table appear first. I.e. B before C before N etc… 2o Elements lower in a group appear first. I.e. I before Br before Cl… 3o Oxygen appears last unless with F. 4o Hydrogen appears between N and Se. B, Si, C, As, P, N, H, Se, Si, I, Br, Cl, O, F Metal and Non-Metal A) Only one oxydation state (Type I) Metal Name + Non-Metal Root Name + “ide” B) Multiple oxydation states (Type II) Metal Name + (Roman Numeral2) + Non-Metal Root Name + “ide” 2 Roman Numeral is equal to oxydation state. C) Multiple oxydation states (Old System) Latin Root of Metal Name3 + Suffix4 + Non-Metal Root Name + “ide” 3 Ex. Cupr (Cu), Plumb (Pb), Ferr (Fe)… 4 Suffix is “-ic” for Highest oxydation State. Suffix is “-ous” for Lowest oxydation State. Mnemonic: Highest = “-ic”; Lowest = “-ous”. Polyatomic Ions containing Oxygen (Prefix +5) Non-Oxygen Element Root + Suffix5 5 Suffixes for Polyatomic Ions: Prefix / Suffix Hypo________ite ________ite ________ate Per________ate Only one combination 2 possible combos Only one oxyion type Least Oxygen Most Oxygen 4 possible combos Least Oxygen 2nd Least Oxygen 2nd Most Oxygen Most Oxygen Acids A) Binary acids (H + Non-metal) and Polyatomic Ion Acids without oxygen Hydro + Non-metal Root Name + “-ic” + Acid B) Polyatomic Ion Acids containing oxygen (H + Polyatomic Ion) Polyatomic Ion Root Name + Suffix6 + Acid 6 Polyatomic Ion Suffix changes from “-ite” to “-ous” or from “-ate” to “-ic”.