Extensive and Intensive Properties
advertisement

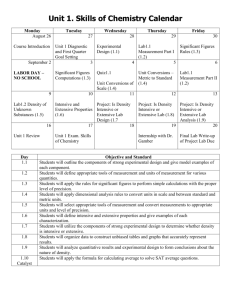
Extensive and Intensive Properties Ck12 Science Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-source, collaborative, and web-based compilation model, CK-12 pioneers and promotes the creation and distribution of high-quality, adaptive online textbooks that can be mixed, modified and printed (i.e., the FlexBook® textbooks). Copyright © 2015 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Commons from time to time (the “CC License”), which is incorporated herein by this reference. Complete terms can be found at http://www.ck12.org/about/ terms-of-use. Printed: June 7, 2015 AUTHOR Ck12 Science www.ck12.org C HAPTER Chapter 1. Extensive and Intensive Properties 1 Extensive and Intensive Properties • Define extensive property. • Define intensive property. • Give examples of extensive and intensive properties How much is twenty dollars really worth? I agree to mow someone’s lawn for twenty dollars (it’s a fairly big yard). When they pay me, they give me a $20 bill. It doesn’t matter whether the bill is brand new or old, dirty, and wrinkled – all these bills have the same value of $20. If I want more $20 bills, I have to mow more lawns. I can’t say, “This particular bill is actually worth more than $20.” To have more money, I have to put in more work. Extensive and Intensive Properties Extensive Properties Some properties of matter depend on the size of the sample, while some do not. An extensive property is a property that depends on the amount of matter in a sample. The mass of an object is a measure of the amount of matter that an object contains. A small sample of a certain type of matter will have a small mass, while a larger sample will have a greater mass. Another extensive property is volume. The volume of an object is a measure of the space that is occupied by that object. The figure below illustrates the extensive property of volume. The pitcher and glass both contain milk. The pitcher holds approximately two quarts and the glass will hold about 8 ounces of milk. The same milk is in each container. The only difference is the amount of milk contained in the glass and in the pitcher Intensive Properties The electrical conductivity of a substance is a property that depends only on the type of substance. Silver, gold, and copper are excellent conductors of electricity, while glass and plastic are poor conductors. A larger or smaller piece of glass will not change this property. An intensive property is a property of matter that depends only on the type of matter in a sample and not on the amount. Other intensive properties include color, temperature, density, and solubility. 1 www.ck12.org FIGURE 1.1 Milk pitcher and glass. The copper wire shown in the picture below has a certain electrical conductivity. You could cut off the small end sticking out and it would have the same conductivity as the entire long roll of wire shown here. The conductivity is a property of the copper metal itself, not of the length of the wire. FIGURE 1.2 Copper wire. Summary • An extensive property is a property that depends on the amount of matter in a sample. • Mass and volume are examples of extensive properties. • An intensive property is a property of matter that depends only on the type of matter in a sample and not on the amount. • Color, temperature, and solubility are examples of intensive properties. Explore More Use the link below to answer the following questions: 2 www.ck12.org Chapter 1. Extensive and Intensive Properties • Physical and Chemical Properties of Matter at https://www.boundless.com/chemistry/textbooks/boundlesschemistry-textbook/introduction-to-chemistry-1/physical-and-chemical-properties-of-matter-28/physical-andchemical-properties-of-matter-181-1817/ . 1. 2. 3. 4. Give examples of extensive properties of matter. Give examples of intensive properties of matter. Distinguish between a chemical and physical property of matter. Describe two chemical properties of matter. Review 1. 2. 3. 4. Define extensive property. Give two examples of extensive properties, Define intensive property. Give two examples of intensive properties. • extensive property: A property that depends on the amount of matter in a sample. • intensive property: A property of matter that depends only on the type of matter in a sample and not on the amount. References 1. Courtesy of the US Mint. http://commons.wikimedia.org/wiki/File:US-Series-1995-$20-Obverse.jpg . 2. Zenon Niewada (Wikimedia: Pitcherman). http://commons.wikimedia.org/wiki/File:Milk_Pitcher_With_Lid .jpg . 3. User:Inductiveload/Wikimedia Commons. http://commons.wikimedia.org/wiki/File:Tinned_Copper_Wire_anaglyph.jpg . 3