Mycobacteria: Part 1
10/15/2014
This is the written version of our Hot Topic video presentation available at:
MayoMedicalLaboratories.com/hot-topics
Welcome to Mayo Medical Laboratories Hot Topics. These presentations provide short
discussion of current topics and may be helpful to you in your practice.
©2014 Medical Foundation for Medical
Education and Research. All rights reserved.
1
Mycobacteria: Part 1
10/15/2014
Our speaker for this program is Dr. Nancy Wengenack, Director of the Mycology and
Mycobacteriology Laboratories and Associate Professor of Laboratory Medicine and
Pathology in the Division of Clinical Microbiology at Mayo Clinic in Rochester,
Minnesota. This is the first of a 3-part series on tuberculosis. In Part 1, Dr. Wengenack
provides an overview of the most appropriate methods for culturing and identifying
mycobacteria.
Thank you, Cara, Today, I will be discussing the use of stains and culture methods for
mycobacteria.
2
Mycobacteria: Part 1
10/15/2014
Prior to beginning this program, I wish to acknowledge the receipt of research support
from Trek Diagnostics.
3
Mycobacteria: Part 1
10/15/2014
The objectives of this Hot Topic are:
• to provide a general overview of the key characteristics of mycobacteria
• to describe the stains useful for mycobacteria
• and to discuss methods for the culture of mycobacteria
4
Mycobacteria: Part 1
10/15/2014
Mycobacteria are slim, slightly curved or rod-shaped bacilli. Technically they are Gram
positive organisms but in reality they do not take up the Gram stain well and this is not
a good method for visualizing mycobacteria. Mycobacteria are obligate aerobes and
they are extremely hardy organisms because they are resistant to dessication and
drying as well as most disinfectants. Some mycobacteria have extremely slow growth
rates with generation times of 12-24 hours. If you recall, E. coli has a generation time
of about 20 minutes so you will see a colony of E. coli on a culture plate within a day or
so of plating whereas colonies of the slowly growing mycobacteria can take several
days or even several weeks before they are visible on a culture plate.
5
Mycobacteria: Part 1
10/15/2014
The reason that mycobacteria are such hardy organisms is due to their unique cell wall
structure. The mycobacterial cell wall is nearly 60% lipid consisting of waxes and
unique long-chain fatty acids known as mycolic acids. This high lipid content contrasts
with other Gram positive bacteria which contain approximately 5% lipids and with
Gram negative bacteria which have approximately 20% lipids. Mycolic acids make the
mycobacterial cell surface extremely hydrophobic and resistant to staining with basic
aniline dyes such as the Gram stain and resistant to penetration by many of the drugs
that are used to treat infections caused by other bacteria.
6
Mycobacteria: Part 1
10/15/2014
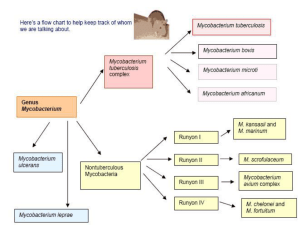
Currently there are approximately 150 recognized species of mycobacteria. Many have
been implicated as the cause of human disease but some are strictly environmental
organisms. The most well-known mycobacterium is Mycobacterium tuberculosis which
is the causative agent of tuberculosis. Mycobacterium tuberculosis complex is the
name used to describe a very closely related group of mycobacteria which cause TB
disease in a variety of species and which are very difficult to distinguish using
methodology commonly available in most clinical microbiology laboratories.
Specialized methods targeting multiple loci are generally needed to distinguish
between members of the M. tuberculosis complex. The members of the
Mycobacterium tuberculosis complex include Mycobacterium tuberculosis which
causes tuberculosis in humans, Mycobacterium bovis which cause tuberculosis in
cattle and occasionally in humans, Mycobacterium bovis BCG which is the vaccine
strain used in many parts of the world other than the U.S., Mycobacterium africanum
which is occasionally seen in humans and there are a few other uncommon species
which generally cause tuberculosis-like disease in animals.
7
Mycobacteria: Part 1
10/15/2014
In addition to Mycobacterium tuberculosis complex, the remaining species of
mycobacteria are referred to as nontuberculous mycobacteria which you will see
abbreviated as NTMs or as MOTTs which is an abbreviation for mycobacteria other
than tuberculosis. The nontuberculous mycobacteria can be further subdivided into
those that grow slowly and require greater than 7 days to form mature colonies on
solid medium and those that grow rapidly and form mature colonies on solid medium
within 7 days. In addition, there is one mycobacterium species, Mycobacterium leprae,
which cannot be grown in laboratory culture on agar plates. Mycobacterium leprae is
the causative agent of leprosy and there are molecular methods available for the
detection and identification of this species.
Examples of slowly growing nontuberculous mycobacteria include Mycobacterium
avium complex, Mycobacterium gordonae and Mycobacterium kansasii. Examples of
rapidly growing nontuberculous mycobacteria include Mycobacterium fortuitum,
Mycobacterium chelonae, Mycobacterium abscessus and Mycobacterium smegmatis.
Nontuberculous mycobacteria can be further subdivided using the Runyon
classification which was described by the microbiologist Ernest Runyon in 1959.
Runyon utilized differences in growth rate and pigmentation to divide mycobacteria
into the slowly growing and rapidly growing classification. Within the slowly growing
8
Mycobacteria: Part 1
10/15/2014
mycobacteria, he subdivided the species into the nonphotochromogens, the
photochromogens and the scotochromogens.
8
Mycobacteria: Part 1
10/15/2014
The nonphotochromogens are nontuberculous mycobacteria which are not pigmented
regardless of their exposure to light. On the culture plate in this slide, you see a
mycobacteria without pigment regardless of whether it is grown in the dark as shown
on the plate on the left or whether it is grown in the light as shown on the plate on the
right.
9
Mycobacteria: Part 1
10/15/2014
Scotochromogens are nontuberculous mycobacteria that are always pigmented. They
are pigmented when grown in the absence of light and pigmented when grown with
exposure to light. Examples include Mycobacterium scrofulaceum, Mycobacterium
gordonae and Mycobacterium szulgai.
10
Mycobacteria: Part 1
10/15/2014
Photochromogens are nontuberculous mycobacteria which are non-pigmented in the
absence of light as shown on the culture plate on the left-hand side of this slide but
which develop pigmentation when exposed to light as shown in the right-hand side of
this slide. Examples include Mycobacterium kansasii and Mycobacterium marinum.
11
Mycobacteria: Part 1
10/15/2014
Over the course of the next few slides, we will review and discuss the specialized stains
available for mycobacteria.
12
Mycobacteria: Part 1
10/15/2014
When submitting specimens for direct microscopic examination and culture for
mycobacteria, virtually any specimen type can be submitted. The most common
sources are the respiratory specimens but tissue, body fluids, urine, and stool are
other specimens encountered commonly in the laboratory. For respiratory specimens,
it is recommended that 3 sputum specimens be submitted for acid-fast bacilli smear
and mycobacterial culture. First morning specimens are generally preferred because it
is felt the acid-fast bacilli have become more concentrated in the sputum as the
patient sleeps overnight and this improves sensitivity of the smear. Collecting sputa on
three separate days is also recommended to improve sensitivity. For children who
often have difficulty producing a quality sputum specimen, gastric washes can be
collected and submitted as an alternative.
The specimen must be collected and transported to the laboratory in a sterile, sealed,
leak-proof container. Exposure to domestic and hospital water sources (even as a
rinse) should be avoided since mycobacteria can be found in these environments.
13
Mycobacteria: Part 1
10/15/2014
As mentioned earlier, mycobacteria do not stain well with the Gram stain used for
other bacteria. In this slide you can see a specimen stained with the Gram stain and
there are a number of mycobacteria bacilli present which are difficult to see but they
are circled. If you look closely, you can see faint purple rods or white, ghosting
outlines of rods which are the mycobacteria. This failure to stain well with the Gram
stain is due to the highly lipophilic cell wall that we discussed earlier. The long, chain
fatty acids in the mycobacterial cell wall make the organism very resistant to staining
with many dyes including the Gram stain dye.
14
Mycobacteria: Part 1
10/15/2014
Mycobacteria are known as “acid-fast” bacilli or AFB for short. They are called this
because a complex is formed between the mycolic acid in the mycobacterial cell wall
and specific dyes which include carbol-fuchsin dye or Auramine-O dye. Auramine O is
a fluorescent dye. The formation of a physical complex between the dye and the
mycolic acid renders the mycobacteria resistant to destaining by mineral acid and this
is why mycobacteria are referred to as acid-fast bacteria or AFB. After destaining with
mineral acid, a counter stain is applied to aid with visualization of any non-acid fast
bacteria present. So mycobacteria retain the carbol-fuchsin or Auramine O dye while
other bacteria do not.
15
Mycobacteria: Part 1
10/15/2014
This slide shows a Ziehl-Neelsen stained specimen. Ziehl-Neelsen is a common acidfast stain which utilizes heat to aid in penetration of the carbol-fuchsin dye. Another
variation on this method is called the Kinyoun method which utilizes phenol to aid
penetration of the carbol-fuchsin dye. Either method results in the acid-fast bacteria
being visualized by staining red while background and non-acid fast bacteria are
stained blue by the counterstain.
16
Mycobacteria: Part 1
10/15/2014
This slide shows an Auramine-rhodamine fluorescent stain with small orange or apple
green bacilli seen fluorescing at 400 X magnification on the left and small clumps of
acid-fast rods seen fluorescing at 1000X under oil on the right.
17
Mycobacteria: Part 1
10/15/2014
Another stain useful for mycobacteria is the Fite stain which is a modification of the
Ziehl-Neelsen stain that uses a milder decolorizing agent that is thought to work better
for some of the less hardy mycobacteria. Fite stain is most often used in Pathology but
the tissue processing that occurs in Pathology laboratories can at times damage the
mycolic acids in the mycobacterial cell wall making it difficult to find AFB regardless of
the stain used.
LED or Light-Emitting Diode microscopy is gaining traction in developing countries
where fluorescent microscopes are scare or too expensive to obtain. LED microscopes
can run on batteries when a constant power source isn’t available and a World Health
Organization study indicated that LED microscopy was superior to Zeehl-Neelsen stain
and equivalent to a fluorescent stain. The WHO has recommended the replacement of
fluorescence microscopy with LED microscopy.
One important point to remember is that one cannot reliable speciate mycobacteria
using microscopy. Mycobacterium tuberculosis looks like Mycobacterium avium which
looks like Mycobacterium abscessus. Additional testing is needed to reliably speciate
mycobacteria and we will discuss that in our next Hot Topic presentation.
Another important point to remember is that a positive acid-fast smear suggests a
18
Mycobacteria: Part 1
10/15/2014
higher likelihood of infectivity if the patient has pulmonary tuberculosis.
18
Mycobacteria: Part 1
Acid fast smears are helpful if positive but their biggest drawback is that they are not
terribly sensitive. The data on this slide is from the Minnesota Department of Health
for the years 2008 through 2012 and it shows that of 491 culture-confirmed cases of
tuberculosis, only 40% of the cases had a positive acid-fast smear. So a negative acidfast smear does not rule out tuberculosis or any other mycobacterial disease.
10/15/2014
Mycobacteria: Part 1
10/15/2014
Is it better to do two or three acid fast smears instead of a single smear? The data
summarized on this slide from several studies over the past 20 years suggests that it is.
You can see from the slide that a single smear has a sensitivity of 66 to 89% depending
on the study. Factors such as the patient population, the experience of the reader, and
the bacillary load all contribute to smear sensitivity. The data also demonstrate that
an additional 5 to 24% sensitivity is added by performing a second smear while 5-10%
is added by performing a third smear. So this slide provides data which supports the
common practice of obtaining up to 3 serial acid fast smears on a patient. More than
3 smears within a close timeframe is not necessarily better though and the gain in
sensitivity drops off so the value of serial smears beyond 3 is questionable.
20
Mycobacteria: Part 1
10/15/2014
This slide provides data to address the question of why early morning sputum
specimens preferred for acid-fast smears. The two recent studies cited here
demonstrated that collecting random or spot sputum specimens for acid fast smear
has a sensitivity of 43-57% while collecting early morning sputum specimens lead to
an increased sensitivity of 65-100% depending on the study. So, as conventional
wisdom suggests, early morning sputum specimens have increased sensitivity for acidfast smears over randomly collected sputum specimens.
21
Mycobacteria: Part 1
10/15/2014
Now we will move from acid-fast smears to discussing culturing of mycobacteria.
22
Mycobacteria: Part 1
10/15/2014
An important point to remember about the culturing of mycobacteria is that nonsterile specimens submitted for mycobacterial cultures often contain other bacteria
which grow more rapidly than mycobacteria and which will outpace or overgrow any
mycobacteria which may be present in the specimen. Therefore it is important to
perform pre-culture processing of specimens to reduce the amount of bacteria other
than mycobacteria which may be present. Non-sterile specimen sources can be pretreated with a variety of agents but the most common are N-acetyl cysteine and
sodium hydroxide. N-acetyl cysteine is a mucolytic agent which helps to break up any
mucus present in respiratory specimens and aids in releasing bacteria including
mycobacteria so they can access the nutrients provided by the culture medium.
Sodium hydroxide, often used in a final concentration of 2%, is a decontaminating
agent which kills competing bacteria while leaving the mycobacteria viable for culture.
Maintaining strict time limits for exposure to the sodium hydroxide is important
because the mycobacteria are generally more hardy than other bacteria but even they
will be rendered non-viable if exposed to sodium hydroxide for too long.
After the digestion and decontamination steps, the specimen is spun down to pellet
the mycobacteria and rehydrated with a minimum volume of phosphate buffered
saline before being plated on culture medium.
23
Mycobacteria: Part 1
10/15/2014
The sensitivity of mycobacterial culture is much better than the sensitivity of acid fast
smear. For an acid-fast smear microscopy, approximately 104 to 105 acid fast bacilli per
milliliter of specimen are needed for a positive smear. Only 10-100 viable organisms
per milliliter of specimen are required for a positive mycobacterial culture.
Mycobacterial cultures are performed using both solid medium and broth-based liquid
medium. The two types of solid medium utilized most often are egg-based
Lowenstein-Jensen, commonly referred to as “LJ” medium and an agar-based medium
known as Middlebrook medium. Broth-based medium is also utilized because it
generally provides a more rapid time to positivity for mycobacterial cultures. Solid
medium can on average 15-30 days for growth to appear while the use of a broth
medium reduces that average time to positivity to 10 days on average. There are two
FDA-cleared commercial platforms for the broth-based culture of mycobacteria and
those are the BACTEC Mycobacterial Growth Indicator Tube system, commonly called
the MGIT system, and the VersaTREK system.
24
Mycobacteria: Part 1
10/15/2014
On the next slide, I show the typical colony morphology of Mycobacterium tuberculosis
growing in culture on Lowenstein-Jensen medium. Note that the colonies are
roughened and tan colored or as they are commonly known, “rough and buff”.
25
Mycobacteria: Part 1
10/15/2014
The next slide shows the BACTEC MGIT 960 rapid broth culture system for mycobacteria.
The instrument is called the 960 because each of the three drawers holds 320 tubes or
960 tubes total. A close up of the inside of a drawer is shown on the right and you can see
that each space in the drawer holds a test tube which contains Middlebrook broth
medium. In addition, each tube contains a fluorescent indicator in the bottom. The
fluorescence is quenched by the presence of oxygen in the medium inside the tube. After
addition of a patient’s specimen to the tube, any bacteria present, including mycobacteria,
will use up the oxygen in the tube and the fluorescent indicator will signal that an
organism is growing in the medium. The system is semi-automated and continuously
monitored such that once the oxygen in the tube is depleted and the fluorescent indicator
signals, a laboratory technologist can remove the tube from the instrument and can begin
the task of identifying any mycobacteria present.
26
Mycobacteria: Part 1
10/15/2014
This slide pictures the other FDA-cleared, semi-automated system for the culture of
mycobacteria, the VersaTREK system. This system utilizes the small bottles filled with
sponges and Middlebrook broth medium as shown in the bottom right hand picture on
this slide. The sponges are purported to increase the surface area and enhance the
growth of the mycobacteria. The system detects the growth of bacteria including
mycobacteria in the bottles by sensing changes in headspace pressure as organisms
grow in the medium. When the pressure changes past the set threshold level, the
instrument signals and a laboratory technologist can remove the bottle from the
instrument and can begin the task of identifying any mycobacteria present.
27
Mycobacteria: Part 1
10/15/2014
The pie chart on this slide summarizes data from the Minnesota Department of
Health for 806 clinically-diagnosed cases of tuberculosis for the years 2008
through 2012. Of these 806 cases, 75% had a positive mycobacterial culture
which is significantly higher than the proportion that had a positive acid fast
smear shown in the earlier slide. It is important to note that although the
sensitivity of mycobacterial culture is better than that of acid-fast smear, there
are still clinically-confirmed cases of tuberculosis which do not produce a
positive culture. There are a variety of reasons for this but some may include
sampling variability or the use of antibacterial agents which have some activity
against M. tuberculosis prior to obtaining a sample for mycobacterial culture.
28
Mycobacteria: Part 1
10/15/2014
In summary, mycobacteria are environmental organisms found most often in soil and
water sources. Many are proven human pathogens but only Mycobacterium
tuberculosis complex and Mycobacterium leprae are thought to be transmitted from
person-to-person.
The number of recognized species of mycobacteria continues to grow with
approximately 150 currently valid species reported.
Mycobacteria are known as “acid-fast” organisms because of their unique cell wall
which forms a complex with carbol-fuchsin or Auramine O dyes. This complex aids in
retaining the dye in the presence of an acidic decolorizing agent. Other bacteria lack
the mycolic acids in their cell walls and therefore do not retain the dyes in the
presence of an acidic decolorizing agent.
Multiple specimens and specialized processing steps are often needed for the recovery
of mycobacteria in culture. The use of both broth and solid culture medium provides
optimal recovery.
29
Mycobacteria: Part 1
10/15/2014
I thank you listening to this Hot Topic on mycobacterial stains and cultures. I hope you
will join me for the second installment in this series which will discuss methods for the
detection and identification of Mycobacterium tuberculosis complex.
30
Mycobacteria: Part 1
10/15/2014
31