Parekh - Imiquimod Study
advertisement

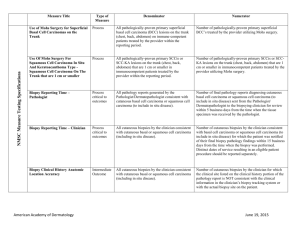
Imiquimod 5% Cream as an Adjunctive Therapy for Primary Nodular Nasal Basal Cell Carcinomas Prior to Mohs Surgery: A Randomized, Vehicle-Controlled, DoubleBlind Study Palak Parekh, MD David F. Butler, MD Disclosures • Investigator initiated study • Grant from 3M and Graceway pharmaceuticals • Grant from Scott and White Education and Research Division Scott and White Clinic Temple, Texas Imiquimod for BCC Imiquimod: Mechanism of Action • • • • • Immune response modifier Stimulates Toll-like receptors 7 and 8 Induces IFN-α, IL-1, -6, -8, -10, -12, TNF-α Recruits inflammatory cells Increased expression of Notch 1 and Fas receptor on BCCs • Decreased expression of bcl-2 • 2004 FDA approval for superficial BCCs on trunk and extremities • Applied 5x per week for 6 weeks • Histologic cures: 82% (5x per week) 79% (7x per week) (Giesse J et al.) Imiquimod for Nodular BCCs Nodular BCC Studies have shown: Study • Clearance rates less for nodular BCCs (65-76%) than superficial BCCs • Clearance dependent on frequency and duration of treatment • Studies not confined to the face • Histologic confirmation often lacking Sterry Eigentler Peris Shumack Frequency Duration 3x/week 3x/week 3x/week 3x/week 5x/week 7x/week 6 weeks 8 weeks 12 weeks 12 weeks 12 weeks 12 weeks Clearance 65% 64%(histo) 52.6% 60% 70% 76% 1 Nasal BCCs • • • • • Most common site for BCC (25-30%) Cosmetically important Indication for Mohs surgery Surgical defects often require flap or graft Imiquimod could be used as adjunctive therapy in combination with surgery Hypothesis Nightly application of imiquimod 5% cream with occlusion for 6 weeks prior to Mohs surgery should reduce the number of stages required, size of the defect, and cost of Mohs surgery and wound repair for primary nodular basal cell carcinomas of the nose. Study Design Study Characteristics • Prospective, randomized, double-blind, vehiclecontrolled study • Adults, non-pregnant, immunocompetent • Primary nodular nasal basal cell carcinoma • < 1cm in size • 2 mm punch biopsy histological confirmation • Nightly application of vehicle or imiquimod 5% cream for 6 weeks • Covered with small band-aid • Mohs surgery performed 10 weeks after starting study (4 weeks off treatment prior to Mohs) • 31 patients entered study • 28 patients completed study • 3 in Imiquimod group discontinued study: 2 local adverse events, 1 other illness • Vehicle: 16 patients: 9 male, 7 female age: 75.31 + 11.38 • Imiquimod: 12 patients: 8 male, 4 female age: 73.27 + 10.53 • No significant difference in baseline characteristics. Adverse Events Primary and Secondary Outcomes • Number of Mohs stages for clear margins • If 1st stage clear, presence or absence of tumor in block (# of complete responders) • Surgical defect size • Cost of Mohs stages in RVUs • Cost of wound repair in RVUs Vehicle (n=16) Local Adverse event (redness, blisters, erosions, crusting) Systemic symptoms (flu-like symptoms) Pain 3wk 4 6wk 2 (p=.0005) 1(nausea) 0 Imiquimod (n=13) 10 10 0 1 2 Study Outcome Vehicle (n=16) Study Outcome Imiquimod (n=12) ITT (n=15) 11 (68.8%) 11 (91.7%) 11 (73.3%) 5 (31.3%) 1 (8.3%) 4 (26.7%) (p=0.20) (p=1.0) Number of Mohs stages: One stage only Two stages Vehicle Imiquimod (n=11) (n=11) yes Study Outcome Defect size: (mean) Presence of tumor in block if first stage had clear margins: Vehicle 100.30 mm sq. + 41.90 9 (81.2%) 6 (54.55%) no 2 (18.18%) 5 (45.45%) (P=0.36) 6 cases with residual carcinoma in imiquimod arm added to 1 case requiring second stage = 7/12 (58.3%) cases had persistent tumor. Thus, only 5/12 (41.7%) in imiquimod arm were “complete responders.” Study Outcome Imiquimod 88.47 mm sq. + 27.82 Vehicle (n=16) Cost of Mohs surgery in RVUs: Imiquimod (n=12) 8.45 7.83 + 1.28 + 0.82 (p=0.70) (p=0.12) Study Outcome Vehicle (n=16) Cost of wound repair in RVUs: 8.59 + 2.96 ( p=0.62) Conclusions Imiquimod (n=12) 8.47 + 3.39 • Our study did not demonstrate a statistically significant difference in the number of Mohs stages, size of the mean surgical defect, and cost of Mohs surgery and reconstructive repair between the treatment and vehicle group. • Limitation of our study was small sample size. Sample size of 56 would have been needed for sufficient power to demonstrate statistically significant difference. • A larger study with possibly varied histologic types of basal cell carcinomas may aid in demonstration of statistical significance. 3 Bibliography Conclusions • The low percentage of histologic “complete responders” (42%) compared to previous studies on superficial BCCs (79-82%) warrants caution when using imiquimod as a single modality for treatment of nodular BCCs on the nose. • Local inflammatory reactions associated with imiquimod may limit its usefulness as an adjunctive treatment. 1. Geisse JK, Rich P, Pandya A, et al. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: a double-blind, randomized, vehicle-controlled study. J Am Acad Dermatol. 2002 47(3): 390-398. 2. Sterry W, Ruzicka T, Herrera E, et al. Imiquimod 5% cream for the treatment of superficial and nodular basal cell carcinomas: randomized studies comparing lowfrequency dosing with and without occlusion. Br J Dermatol. 2002; 147: 12271236. 3. Peris K, Campione E, Micantonio T, et al. Imiquimod treatment of superficial and nodular basal cell carcinoma: 12-week open-label trial. Dermatol Surg. 2005; 31(3): 318-323. 4. Shumack S, Robinson J, Kossard S, et al. Efficacy of topical 5% imiquimod cream for the treatment of nodular basal cell carcinoma. Arch Dermatol. 2002; 138(9): 1165-1171. 5. Eigentler TK, Kamin A, Weide BM, et al. A phase III, randomized, open label study to evaluate the safety and efficacy of imiquimod 5% cream applied thrice weekly for 8 and 12 weeks in the treatment of low-risk nodular basal cell carcinoma. J Am Acad Dermatol. 2007; 57(4): 616-621. 6. Torres A, Niemeyer A, Berkes B, et al. 5% imiquimod cream and reflectancemode confocal microscopy as adjunct modalities to mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg. 2004; 30(12 pt 1): 1462-1469. 7. Huber A, Huber JD, Skinner RB Jr, et al. Topical imiquimod treatment for nodular basal cell carcinomas: an open-label series. Dermatol Surg. 2004; 30(3): 429430 4